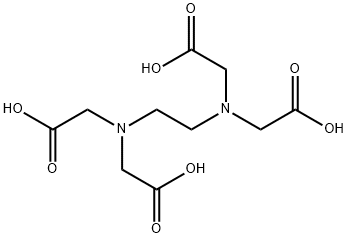
Этилендиаминтетрауксусная кислота
- английское имяEthylenediaminetetraacetic acid
- CAS №60-00-4
- CBNumberCB9853940
- ФормулаC10H16N2O8
- мольный вес292.24
- EINECS200-449-4
- номер MDLMFCD00003541
- файл Mol60-00-4.mol
| Температура плавления | 250 °C (dec.) (lit.) |
| Температура кипения | 434.18°C (rough estimate) |
| плотность | 1.46 g/cm3 at 20 °C |
| давление пара | <0.013 hPa (20 °C) |
| показатель преломления | n |
| Fp | >400°C DIN 51758 |
| температура хранения | 2-8°C |
| растворимость | 3 M NaOH: 100 mg/mL |
| пка | pKa 2 (Uncertain);10.26 (Uncertain) |
| форма | crystalline |
| цвет | White to almost white |
| Запах | Odorless |
| Водородный показатель | 2.5 at 10 g/l at 23 °C |
| РН | 2.5 (10g/l, H2O, 23℃)(slurry) |
| Растворимость в воде | 0.5 g/L (25 ºC) |
| λмакс | λ: 280 nm Amax: ≤0.25 |
| Разложение | 240 °C |
| Мерк | 14,3517 |
| БРН | 1716295 |
| Стабильность | Stable. Incompatible with copper, copper alloys, nickel, aluminium, strong oxidizing agents, strong bases |
| LogP | -0.836 (est) |
| Непрямые добавки, используемые в веществах, контактирующих с пищевыми продуктами | ETHYLENEDIAMINETETRAACETIC ACID |
| Справочник по базе данных CAS | 60-00-4(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 2 |
| Словарь онкологических терминов NCI | edetic acid; EDTA |
| FDA UNII | 9G34HU7RV0 |
| Справочник по химии NIST | N,N'-1,2-Ethane diylbis-(N-(carboxymethyl)glycine)(60-00-4) |
| Система регистрации веществ EPA | Ethylenediaminetetraacetic acid (60-00-4) |
| Информация о косметике | EDTA |
| Коды опасности | Xi | |||||||||
| Заявления о рисках | 36-52/53-36/37/38-36/38 | |||||||||
| Заявления о безопасности | 26-61-37/39-36 | |||||||||
| РИДАДР | UN 3077 9 / PGIII | |||||||||
| WGK Германия | 2 | |||||||||
| RTECS | AH4025000 | |||||||||
| F | 3 | |||||||||
| Температура самовоспламенения | >200 °C | |||||||||
| TSCA | Yes | |||||||||
| кода HS | 2922 49 85 | |||||||||
| Банк данных об опасных веществах | 60-00-4(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 orally in Rabbit: 2580 mg/kg | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H319:При попадании в глаза вызывает выраженное раздражение.
-
оператор предупредительных мер
P264:После работы тщательно вымыть кожу.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
P337+P313:Если раздражение глаз не проходит обратиться за медицинской помощью.
Этилендиаминтетрауксусная кислота химические свойства, назначение, производство
Описание
Ethylenediaminetetraacetic Acid (EDTA) is a common polydentate ligand. In EDTA, the hydrogen atoms are easily removed in solution to produce anionic EDTA4-. In its anionic form Ethylenediaminetetraacetic Acid (EDTA) has six binding atoms, two nitrogen and four oxygen.Ethylenediaminetetraacetic Acid (EDTA) binds to a metal ion at the six binding sites, wrapping itself around the metal ion, forming a very stable complex.the strong grasp of Ethylenediaminetetraacetic Acid (EDTA) on the metal ion is analogous to a crab or lobster clamping down on an object with its claw, hence the name chelation. Ethylenediaminetetraacetic Acid (EDTA) is such an effective chelating agent because it can deactivate a metal at up to six sites.
Химические свойства
Ethylenediaminetetraacetic acid is a solid.История
Ethylenediaminetetraacetic Acid (EDTA) was first synthesized in the early 1930s by the German chemist Ferdinand Münz working for I. G. Farben. Münz, who was looking for a substitute for citric acid to use with dye solutions in the textile industry, was the first to patent a process for Ethylenediaminetetraacetic Acid (EDTA) synthesis in Germany in 1935. Münz subsequently applied for United States patents in 1936 and 1937 (U.S. Patent Number 2130505); his method involved reacting monochloroacetic acid (C2H3ClO2) and ethylene diamine (C2H8N2). Concurrent with Münz’s work, Frederick C. Bersworth in the United States synthesized Ethylenediaminetetraacetic Acid (EDTA) using different methods that gave greater yields and made EDTA’s commercial production economically viable. Bersworth syntheses involved reacting formaldehyde, amines, and hydrogen cyanide. Bersworth and Münz obtained patents for Ethylenediaminetetraacetic Acid (EDTA) production in the 1940s (U.S. Patent Numbers 2407645 and 2461519).Использование
antispasmodicОпределение
An organic chelating agent.Методы производства
Edetic acid may be prepared by the condensation of ethylenediamine with sodium monochloroacetate in the presence of sodium carbonate. An aqueous solution of the reactants is heated to about 90°C for 10 hours, then cooled, and hydrochloric acid is added to precipitate the edetic acid.Edetic acid may also be prepared by the reaction of ethylenediamine with hydrogen cyanide and formaldehyde with subsequent hydrolysis of the tetranitrile, or under alkaline conditions with continuous extraction of ammonia.
Общее описание
Ethylenediamine tetraacetic acid is a colorless crystalline solid. Ethylenediaminetetraacetic acid is slightly soluble in water. The primary hazard is the threat to the environment. Immediate steps should be taken to limit its spread to the environment. Ethylenediaminetetraacetic acid is used in chemical analysis, to make detergents and cleaning compounds, and for many other uses.Реакции воздуха и воды
Slightly soluble in water.Сельскохозяйственное использование
EDTA is short for ethylenediamhetetraacetic acid, an amino polycarboxylic acid. It is a tetraprotic acid and is represented as H4Y with four carboxyl groups and two nitrogen atoms acting as ligand sites. Thus the compound is a hexadentate ligand. Ligands include ions such as Cl-, NO2-and CN- or neutral molecules like NH3 and H2O, which possess a lone pair of electrons that can be shared with a metal cation in coordinate covalent bonds.The water solubility of EDTA is very low and, therefore, its di-sodium salt Na2H2Y.2H2O is commonly used in titrations. The Y4- forms very stable, one-to-one complexes with practically every metal ion in the Periodic Table. The reactions are carried out in a neutral or alkaline medium as the complex decomposes in acidic medium.
(and hence deterioration) of the food product, (d) to increase the storage life of whole blood by removing free calcium ions (Ca2+) to inhibit clotting, and (e) for extracting trace elements, especially copper. EDTA metal complexes, such as NaFeEDTA, MnEDTA, ZnEDTA and CuEDTA are used as fertilizers and foliar sprays.
Биологическая активность
Chelating agent; sequesters di- and trivalent metal ions.Профиль безопасности
Poison by intraperitoneal route. Experimental teratogenic and reproductive effects. Mutation data reported. A general-purpose chelaung and complexing agent. When heated to decomposition it emits toxic fumes of NOx.Безопасность
Edetic acid and edetates are widely used in topical, oral, and parenteral pharmaceutical formulations. They are also extensively used in cosmetics and food products.Edetic acid is generally regarded as an essentially nontoxic and nonirritant material, although it has been associated with doserelated bronchoconstriction when used as a preservative in nebulizer solutions. It has therefore been recommended that nebulizer solutions for bronchodilation should not contain edetic acid.
Edetates, particularly disodium edetate and edetate calcium disodium, are used in a greater number and variety of pharmaceutical formulations than the free acid.
Disodium edetate, trisodium edetate, and edetic acid readily chelate calcium and can, in large doses, cause calcium depletion (hypocalcemia) if used over an extended period or if administered too rapidly by intravenous infusion. If used in preparations for the mouth, they can also leach calcium from the teeth. In contrast, edetate calcium disodium does not chelate calcium. Edetate calcium disodium is nephrotoxic and should be used with caution in patients with renal impairment.
The WHO has set an estimated acceptable daily intake for disodium edetate in foodstuffs at up to 2.5 mg/kg body-weight.
LD50 (mouse, IP): 0.25 g/kg
LD50 (rat, IP): 0.397 g/kg
Возможный контакт
EDTA is a white, odorless, crystalline material or white powderЭкологическая судьба
EDTA can be very persistent in water, including wastewatertreatment plants. EDTA is often found in the receiving waters of many industrial areas, thus being classified as one of the major organic pollutants discharged in waters. The available ecotoxicity data for EDTA indicate that these compounds are slow to degrade under typical environmental conditions but are not expected to bioconcentrate. EDTA compounds range from practically nontoxic to moderately toxic on an acute basis, depending on the salt. Algae and invertebrates are among the most sensitive species based on predictive modeling for acute and chronic endpoints for EDTA, depending on the compound. EDTA and its salts also do not appear to be very toxic for terrestrial wild mammals, and adverse effects from reasonably expected agricultural uses are not expected.хранилище
Although edetic acid is fairly stable in the solid state, edetate salts are more stable than the free acid, which decarboxylates if heated above 150°C. Disodium edetate dihydrate loses water of crystallization when heated to 120°C. Edetate calcium disodium is slightly hygroscopic and should be protected from moisture.Aqueous solutions of edetic acid or edetate salts may be sterilized by autoclaving, and should be stored in an alkali-free container.
Edetic acid and edetates should be stored in well-closed containers in a cool, dry place.
Перевозки
UN3082 Environmentally hazardous substances, liquid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name RequiredМетоды очистки
Dissolve EDTA in aqueous KOH or ammonium hydroxide, and precipitate it twice with dilute HCl or HNO3. Boil it twice with distilled water to remove mineral acid, then recrystallise it from water or dimethylformamide. Dry it at 110o. It also recrystallises from boiling 1N HCl; wash the crystals with distilled H2O and dry them in vacuo. [Ma & Ray Biochemistry 19 751 1980, Beilstein 4 IV 2449.]Несовместимости
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, copper, copper alloys, and nickelРегуляторный статус
Included in the FDA Inactive Ingredients Database (oral, otic, rectal, and topical preparations; submucosal injection preparations). Included in nonparenteral medicines licensed in the UK. Included in the Canadian List of Acceptable Non-medicinal Ingredients.Этилендиаминтетрауксусная кислота запасные части и сырье
сырьё
1of4
запасной предмет
- Ethylenediaminetetraacetic acid tetrasodium salt dihydrate 99.0-102.
- Натрия салицилат
- L-тирозин
- N-альфа-BOC-N-эпсилон-(2-хлор-Z)-L-лизин
- H-LYS(2-CL-Z)-OH
1of3
Этилендиаминтетрауксусная кислота поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +86-18168774353 | China | 248 | 58 | ||
| +8613063595538 | China | 9365 | 58 | ||
| +86-17531190177; +8617531190177 |
China | 6011 | 58 | ||
| +862156762820 +86-13564624040 |
China | 6930 | 58 | ||
| +86-19930503253; +8619930503252 |
China | 5838 | 58 | ||
| +86-17331933971 +86-17331933971 |
China | 2503 | 58 | ||
| +86-15532196582 +86-15373005021 |
China | 2989 | 58 | ||
| +8617756083858 | China | 994 | 58 | ||
| +86-371-86557731 +86-13613820652 |
China | 20314 | 58 | ||
| +8618740459177 | China | 1142 | 58 |
Этилендиаминтетрауксусная кислота Обзор)
- Фолиевая кислота
- Гиалуроновая кислота
- Кислота лимонная
- Этилендиамин дигидрохлорид
- Этиламин гидрохлорид
- Гликолевая кислота
- Натрия эдетат
- Этилендиаминтетрауксусной кислоты динатриевая сол
- EDTA динатриевая соль дигидрат EP
- Этилендиаминтетрауксусной кислоты дикалия соль дигидра
1of4