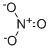
NITRATE
- русский язык имя
- английское имяNITRATE
- CAS №14797-55-8
- CBNumberCB9748190
- ФормулаNO3-
- мольный вес62
- EINECS231-554-3
- номер MDLMFCD00144920
- файл Mol14797-55-8.mol
химическое свойство
| температура хранения | 2-8°C |
| форма | Liquid |
| цвет | Clear colorless |
| Растворимость в воде | Miscible with water. |
| Стандарт первичной питьевой воды EPA | MCL:10,MCLG:10 |
| FDA 21 CFR | 165.110 |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | T93E9Y2844 |
| Система регистрации веществ EPA | Nitrate (14797-55-8) |
NITRATE химические свойства, назначение, производство
Описание
Nitrate is commonly found in drinking water sources especially in agricultural areas where nitrogen fertilizer is used, and where unregulated shallow private wells are more at the risk of contamination. The World Health Organization (WHO) guideline of 50 ppm and the US maximum contaminant level (MCL) of 45 ppm for nitrate in drinking water have been established for protecting infants from methemoglobinemia, commonly known as blue baby syndrome. The health protective value continues to be a subject of public health interest for many years, with varying opinion on whether it is too high or too low. Evaluation of nitrate will need to include consideration of nitrite because both are closely related in the nitrogen cycle in the environment and the body, and nitrite plays a major role in inducing toxicity after its formation from nitrate. More recently, reports of nitrate in drinking water, especially at levels higher than 50 ppm, have been associated with other health effects other than methemoglobinemia. This toxicological review provides an update on the health effects of nitrate with a focus on methemoglobinemia, reproductive and developmental effects, potential carcinogenicity, and especially endocrine/thyroid effects.Химические свойства
A colorless liquid.Использование
In the treatment of angina pectoris; in the manufacture of inorganic and organic nitrates and nitro compounds for fertilizers, dye intermediates, explosives, and many different organic chemicals.Определение
nitrate: A salt or ester of nitric acid.Общее описание
Crystalline solids. Salts of nitrate, such as ammonium nitrate, potassium nitrate, and sodium nitrate.Реакции воздуха и воды
Most are water soluble.Профиль реактивности
Mixtures of metal/nonmetal nitrates with alkyl esters may explode, owing to the formation of alkyl nitrates; mixtures a nitrate with phosphorus, tin (II) chloride, or other reducing agents may react explosively [Bretherick 1979. p. 108-109].Опасность
Moderately toxic.Угроза здоровью
Inhalation, ingestion or contact (skin, eyes) with vapors or substance may cause severe injury, burns or death. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may cause pollution.Пожароопасность
These substances will accelerate burning when involved in a fire. Some may decompose explosively when heated or involved in a fire. May explode from heat or contamination. Some will react explosively with hydrocarbons (fuels). May ignite combustibles (wood, paper, oil, clothing, etc.). Containers may explode when heated. Runoff may create fire or explosion hazard.Экологическая судьба
Nitrate (NO3 -), a product of nitrogen oxidation, is a naturally occurring ion in the environment and integrated into complex organic molecules such as proteins and enzymes required by living systems. Nitrate is a more stable form of oxidized nitrogen than nitrite; however, it can be reduced by microbial action to nitrite, which, in turn, can be reduced to various compounds or oxidized to nitrate by chemical and biological processes. Nitrates occur naturally in soil from microbial oxidation of ammonia derived from organic nitrogenous materials such as plant proteins, animals, and animal excreta. Other source contributions are wastewater, septic tank runoffs, airborne nitrogen compounds emitted by industry and automobiles, nitrogen fertilizer, and manure from animal feeding. Nitrate in groundwater is generally found below 10 ppm, with higher levels in areas of high agricultural activities.NITRATE поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86-15532196582 +86-15373005021 |
China | 3007 | 58 | |
| 18871490254 | CHINA | 28172 | 58 | |
| +86-0371-86658258 +8613203830695 |
China | 30233 | 58 | |
| +86-029-89586680 +86-18192503167 |
China | 7724 | 58 | |
| +86-16631818819 +86-17736933208 |
China | 9300 | 58 | |
| +86 010-65767987 65763930 | China | 23 | 47 | |
| 400-821-8006 | China | 3040 | 75 | |
| 02781293128 | China | 9923 | 55 | |
| 400-1166-196 18502815961 |
China | 14603 | 60 |