AROCLOR 1260, 1X1ML, ISO, 1000UG/ML
- русский язык имя
- английское имяAROCLOR 1260, 1X1ML, ISO, 1000UG/ML
- CAS №11096-82-5
- CBNumberCB9498491
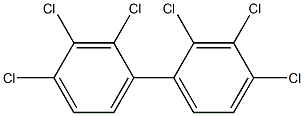
- ФормулаC12H4Cl6
- мольный вес360.87816
- номер MDLMFCD03001820
- файл Mol11096-82-5.mol
| Температура плавления | 115.76°C (estimate) |
| Температура кипения | 457.69°C (rough estimate) |
| плотность | 1.5800 (estimate) |
| давление пара | 130.5 at 25 °C (estimated using GC retention data, Foreman and Bidleman, 1985) |
| показатель преломления | 1.6270 (estimate) |
| Fp | 11 °C |
| температура хранения | room temp |
| растворимость | Soluble in most solvents (U.S. EPA, 1985) |
| Растворимость в воде | 14.4ug/L(20 ºC) |
| констант закона Генри | 0.244, 0.435, 0.754, 1.27, 2.10, 3.36, 5.27, 8.09, and 12.1 at 0.0, 5.0, 10.0, 15.0, 20.0, 25.0, 30.0, 35.5, and 40.0 °C, respectively (predicted, Burkhard et al., 1985) |
| Система регистрации веществ EPA | Aroclor 1260 (11096-82-5) |
| UNSPSC Code | 77101502 |
| NACRES | NA.24 |
| Коды опасности | F,N,Xn,T | |||||||||
| Заявления о рисках | 11-23/24/25-39/23/24/25-67-65-62-51/53-48/20-38-50/53-33-52/53-45 | |||||||||
| Заявления о безопасности | 36/37-45-62-61-60-33-29-16-9-53 | |||||||||
| РИДАДР | 2315 | |||||||||
| WGK Германия | 3 | |||||||||
| Класс опасности | 9 | |||||||||
| Группа упаковки | II | |||||||||
| Банк данных об опасных веществах | 11096-82-5(Hazardous Substances Data) | |||||||||
| Токсичность | Suspected human carcinogen. Acute oral LD50 for rats 1,315 mg/kg (quoted, RTECS, 1985). | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
опасность
-
вредная бумага
H350:Может вызывать раковые заболевания.
H373:Может поражать органы (Нервная система) в результате многократного или продолжительного воздействия при вдыхании.
-
оператор предупредительных мер
P202:Перед использованием ознакомиться с инструкциями по технике безопасности.
P260:Не вдыхать газ/ пары/ пыль/ аэрозоли/ дым/ туман.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P308+P313:ПРИ подозрении на возможность воздействия обратиться за медицинской помощью.
P405:Хранить в недоступном для посторонних месте.
P501:Удалить содержимое/ контейнер на утвержденных станциях утилизации отходов.
AROCLOR 1260, 1X1ML, ISO, 1000UG/ML химические свойства, назначение, производство
Химические свойства
Soft, sticky, light-yellow resin; approx- imately 6.3 chlorine atoms per molecule.Физические свойства
Light yellow, sticky, soft, nonflammable resin with a faint odorИспользование
Secondary plasticizer for polyvinyl chloride; in polyester resins to increase strength of fiberglass; varnish formulations to improve water and alkali resistance; as an insulator fluid for electric condensers and as an additive in very high pressure lubricants. In fluorescent and highintensity discharge ballasts manufactured prior to 1979 (U.S. EPA, 1998).Also used in wire or cable coatings; impregnants in cotton and asbestos braided insulation; coating on glass air filter pads, metal mesh to filter air and gas streams (Monsanto, 1960).
At a concentration of 5 to 25 wt %, increased the effective kill-life of the lindane spray up to 10 times. May have been used in chlordane and BHC insecticide formulations and in melt coatings for paper and cloth. In various nitrocellulose lacquers to impart weather resistance, luster, adhesion, and decreased burning rate. These lacquers may also contain dibutyl phthalate and/or tricresyl phosphate. A typical paper lacquer may contain acetone, isobutyl acetate, ethanol, toluene and up to 20 wt % PCB-1260 (Monsanto, 1960).
Общее описание
Viscous oily liquid.Реакции воздуха и воды
Insoluble in water.Профиль реактивности
Simple aromatic halogenated organic compounds are very unreactive. Reactivity generally decreases with increased degree of substitution of halogen for hydrogen atoms.Угроза здоровью
ACUTE/CHRONIC HAZARDS: Toxic irritant. Hazardous decomposition products.Пожароопасность
Some may burn but none ignite readily. Containers may explode when heated. Some may be transported hot.Профиль безопасности
Confirmed carcinogen with carcinogenic and neoplastigenic data. Moderately toxic by ingestion and skin contact. Experimental reproductive effects. Mutation data reported. When heated to decomposition it emits highly toxic fumes of Cl-. Used in heat transfer, hydraulic fluids, lubricants, and insecticides. See also POLYCHLORINATED BIPHENYLS.Экологическая судьба
Biological. Reported degradation products by the microorganism Alcaligenes BM-2 for a mixture of polychlorinated biphenyls include monohydroxychlorobiphenyl, 2-hydroxy-6-oxochlorophenylhexa- 2,4-dieonic acid, chlorobenzoic acid, chlorobenzoylpropionic acid, chlorophenylacetic acid, and 3-chlorophenyl-2-chloropropenic acid (Yagi and Sudo, 1980). When PCB-1260 was statically incubated in the dark at 25 °C with yeast extract and settled domestic wastewater inoculum, no significant biodegradation was observed (Tabak et al., 1981).Photolytic. PCB-1260 in a 90% acetonitrile/water solution containing 0.2 to 0.3 M sodium borohydride and irradiated with UV light (λ = 254 nm) reacted to yield dechlorinated biphenyls. After 2 h, about 75% of the congeners were destroyed. Without sodium borohydride, only 10% of the congeners had reacted. Products identified by GC include biphenyl, 2-, 3- and 4- chlorobiphenyl, six dichlorobiphenyls, three trichlorobiphenyls, 1-phenyl-1,4-cyclohexadiene, and 1-phenyl-3-cyclohexene (Epling et al., 1988).
Chemical/Physical. Zhang and Rusling (1993) evaluated the bicontinuous microemulsion of surfactant/oil/water as a medium for the dechlorination of polychlorinated biphenyls by electrochemical catalytic reduction. The microemulsion (20 mL) contained didodecyldimethylammonium bromide, dodecane, and water at 21, 57, and 22 wt %, respectively. The catalyst used was zinc phthalocyanine (4.5 nM). When PCB-1260 (100 mg), the emulsion and catalyst were subjected to a current of mA/cm2 on 11.2 cm2 lead electrode for 18 h, a dechlorination yield of >99.8 % was achieved. Reaction products included minor amounts of mono- and dichlorobiphenyls (0.02 mg), biphenyl and reduced alkylbenzene derivatives.
AROCLOR 1260, 1X1ML, ISO, 1000UG/ML поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +1-+1(833)-552-7181 | United States | 57505 | 58 | |
| 010-82848833 400-666-7788 |
China | 94657 | 76 | |
| 025-83697070 | China | 19185 | 64 | |
| 021-021-021-67601398-809-809-809 15221380277 |
China | 9658 | 60 | |
| 400-821-8006 | China | 3040 | 75 | |
| 0755-0755-85201366 18938635012 |
China | 9585 | 58 | |
| +86-400-002-6226 +86-13028896684; |
China | 57423 | 58 | |
| 18017382783 18017382783 |
China | 9611 | 58 | |
| 021-021-61109150 | China | 31121 | 58 | |
| 021-50706066 15221275939 |
China | 30016 | 58 |