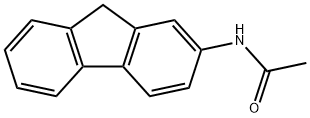
2-АЦЕТАМИДОФЛУОРЕН
- английское имя2-ACETAMIDOFLUORENE
- CAS №53-96-3
- CBNumberCB9463907
- ФормулаC15H13NO
- мольный вес223.27
- EINECS200-188-6
- номер MDLMFCD00001116
- файл Mol53-96-3.mol
| Температура плавления | 192-196 °C(lit.) |
| Температура кипения | 364.56°C (rough estimate) |
| плотность | 1.0707 (rough estimate) |
| показатель преломления | 1.5500 (estimate) |
| температура хранения | Store below +30°C. |
| растворимость | Soluble in acetone, acetic acid, alcohol (Weast, 1986), glycols, and fat solvents (Windholz et al., 1983) |
| форма | Crystalline Powder |
| пка | 14.89±0.20(Predicted) |
| цвет | off-white to tan |
| Растворимость в воде | 10.13 mg/L at 26.3 °C (Ellington et al., 1987) |
| Мерк | 14,4157 |
| БРН | 2807677 |
| Справочник по базе данных CAS | 53-96-3(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | 9M98QLJ2DL |
| Предложение 65 Список | 2-Acetylaminofluorene |
| Система регистрации веществ EPA | 2-Acetylaminofluorene (53-96-3) |
| UNSPSC Code | 12352111 |
| NACRES | NA.25 |
| Коды опасности | T,N | |||||||||
| Заявления о рисках | 45-22-51/53 | |||||||||
| Заявления о безопасности | 53-36/37/39-45 | |||||||||
| РИДАДР | UN 3077 9/PG 3 | |||||||||
| WGK Германия | 3 | |||||||||
| RTECS | AB9450000 | |||||||||
| кода HS | 2924 29 70 | |||||||||
| Класс опасности | 6.1(b) | |||||||||
| Группа упаковки | III | |||||||||
| Банк данных об опасных веществах | 53-96-3(Hazardous Substances Data) | |||||||||
| Токсичность | Acute oral LD50 for mice 1,020 mg/kg (quoted, RTECS, 1985). | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
опасность
-
вредная бумага
H302:Вредно при проглатывании.
H350:Может вызывать раковые заболевания.
-
оператор предупредительных мер
P202:Перед использованием ознакомиться с инструкциями по технике безопасности.
P264:После работы тщательно вымыть кожу.
P270:При использовании продукции не курить, не пить, не принимать пищу.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P301+P312:ПРИ ПРОГЛАТЫВАНИИ: Обратиться за медицинской помощью при плохом самочувствии.
P308+P313:ПРИ подозрении на возможность воздействия обратиться за медицинской помощью.
2-АЦЕТАМИДОФЛУОРЕН химические свойства, назначение, производство
Химические свойства
WHITE TO YELLOW-BROWN CRYSTALLINE POWDERИспользование
N-(2-Fluorenyl)acetamide (2-Acetamidofluorene, 2-AAF), a genotoxic carcinogen, is used to induce liver cancer in animal models such as the 2-AAF/partial hepatectomy rat. 2-AAF may be used to study the mechanism of liver carcinogenesis and as a reference material during its identification or quantitation.Определение
ChEBI: The parent of the class of 2-acetamidofluorenes, being an ortho-fused polycyclic arene that consists of 9H-fluorene bearing an acetamido substituent at position 2. It is a carcinogenic and mutagenic derivative of fluorene.Методы производства
2-Acetylaminofluorene is produced for research purposes only with an estimated U.S. annual usage of less than 20 lb. It was originally developed as a possible insecticide but has never been used for this purpose after discovery of its carcinogenicity. It is now almost exclusively used in the laboratory studies as a model carcinogen and mutagen.Общее описание
White powder or light beige solid.Реакции воздуха и воды
Insoluble in water.Профиль реактивности
2-ACETAMIDOFLUORENE is incompatible with acids, bases and oxidizing agents. Ozone and chlorinating agents oxidize 2-ACETAMIDOFLUORENE .Угроза здоровью
2-Acetylaminofluorene (AAF) is a potent carcinogen in dogs, hamsters, and rats. There is no toxicity information on humans.1Пожароопасность
Flash point data for 2-ACETAMIDOFLUORENE are not available; however, 2-ACETAMIDOFLUORENE is probably combustible.Профиль безопасности
Confirmed human carcinogen with experimental carcinogenic, neoplas tigenic, tumorigenic, and teratogenic data. Moderately toxic by ingestion and intraperitoneal routes. Experimental reproductive effects. Human mutation data reported. When heated to decomposition it emits toxic fumes of NOx.Возможный контакт
2-AAF was intended to be used as a pesticide, but it was never marketed because this chemical was found to be carcinogenic. AAF is used frequently by biochemists and technicians engaged in the study of liver enzymes and the carcinogenicity and mutagenicity ofaromatic amines as a positive control. Therefore, these persons may be exposed to AAF.Канцерогенность
2-Acetylaminofluorene is reasonably anticipated to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in experimental animals.Экологическая судьба
Biological. In the presence of suspended natural populations from unpolluted aquatic systems, the second-order microbial transformation rate constant determined in the laboratory was reported to be 4.8 ± 2.8 x 10-12 L/organism?h (Steen, 1991).Chemical/Physical. Based on first-order rate constants determined at 85.5 °C, hydrolysis halflives at pH values of 2.49, 2.97, 7.34, 9.80, 10.25, and 10.39 were 4.2, 12, 41, 13, 7.2, and 1.9 d, respectively (Ellington et al., 1987). Releases toxic nitrogen oxides when heated to decomposition (Sax and Lewis, 1987).
Перевозки
UN3077 Environmentally hazardous substances, solid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required.Методы очистки
Recrystallise it from toluene (1.3mg in 100mL). Its solubility in H2O is 1.3mg/L at 25o, UV: max nm(log ) : 288(4.43), 313(4.13). [Sawicki J Org Chem 21 271 1956.] It can also be recrystallised from 50% AcOH. [Diels et al. Chem Ber 35 3285 1902]. 9-14C and -14C 2-acetamidofluorene were recrystallised from aqueous EtOH and had m 194-195o and 194o respectively. Potent CARCINOGEN. [Miller et al. Cancer Res 9 504 1949, 10 616 1950, Sadin et al. J Am Chem Soc 74 5073 1952, Beilstein 12 H 3287, 12 IV 3373.]Несовместимости
Hygroscopic. Contact with strong oxidizers may cause fire and explosions. Not compatible with cyanides, acids, and/or acid anhydrides. May form unstable and explosive peroxides; a possible polymerization hazard. Contact with strong oxidizers or strong reducing agents may form flammable gases and cause fire and explosions. A weak base that may react as an acid. Incompatible with strong bases (forming potentially dangerous salts), chlorinated hydrocarbons, nitro compounds. Reacts with azo and diazo compounds, generating toxic gases. Contact with mixture of acetic acid 1 dinitrogen trioxide may cause explosion.Утилизация отходов
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal. Presumably high-temperature incineration with scrubber for any produced nitrogen oxides can be used2-АЦЕТАМИДОФЛУОРЕН запасные части и сырье
2-АЦЕТАМИДОФЛУОРЕН поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| 008657128800458; +8615858145714 |
China | 7738 | 55 | |
| +86 18953170293 | China | 2930 | 58 | |
| +86-0371-86658258 +8613203830695 |
China | 29809 | 58 | |
| +86-0551-65418671 +8618949823763 |
China | 34563 | 58 | |
| 571-88938639 +8617705817739 |
China | 52849 | 58 | |
| +86-89586680 +86-13289823923 |
China | 8670 | 58 | |
| +86-852-30606658 | China | 24727 | 58 | |
| +8617732866630 | China | 18147 | 58 | |
| +86-0576225566889 +86-13454675544 |
China | 12272 | 58 | |
| +8618058761490 | China | 49983 | 58 |