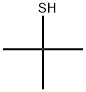
2-метил-2-пропантиол
- английское имя2-Methyl-2-propanethiol
- CAS №75-66-1
- CBNumberCB9432330
- ФормулаC4H10S
- мольный вес90.19
- EINECS200-890-2
- номер MDLMFCD00004857
- файл Mol75-66-1.mol
| Температура плавления | -1.1 °C |
| Температура кипения | 62-65 °C(lit.) |
| плотность | 0.8 g/mL at 25 °C(lit.) |
| плотность пара | 3.1 (vs air) |
| давление пара | 303.5 mm Hg ( 37.7 °C) |
| показатель преломления | n |
| Fp | −12 °F |
| температура хранения | Flammables area |
| растворимость | 1.47g/l slightly soluble |
| форма | Liquid |
| пка | pK1:11.22 (25°C,μ=0.1) |
| цвет | Clear colorless |
| Запах | sulfury |
| Порог?обнаружения?запаха? | 0.000029ppm |
| Растворимость в воде | Slightly soluble in water |
| Мерк | 14,1579 |
| БРН | 505947 |
| Диэлектрическая постоянная | 5.4800000000000004 |
| Стабильность | Stable. Flammable - note low flashpoint. Incompatible with strong oxidizing agents. |
| LogP | 2.206 (est) |
| Справочник по базе данных CAS | 75-66-1(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | 489PW92WIV |
| Справочник по химии NIST | 2-Propanethiol, 2-methyl-(75-66-1) |
| Система регистрации веществ EPA | 2-Propanethiol, 2-methyl- (75-66-1) |
| UNSPSC Code | 12352100 |
| NACRES | NA.22 |
| Коды опасности | F,Xi,Xn,N | |||||||||
| Заявления о рисках | 11-41-65-43-36-51/53 | |||||||||
| Заявления о безопасности | 3-16-26-39-38-36/37-61 | |||||||||
| РИДАДР | UN 2347 3/PG 2 | |||||||||
| WGK Германия | 3 | |||||||||
| RTECS | TZ7660000 | |||||||||
| F | 13 | |||||||||
| Класс опасности | 3.1 | |||||||||
| Группа упаковки | II | |||||||||
| кода HS | 29309090 | |||||||||
| Банк данных об опасных веществах | 75-66-1(Hazardous Substances Data) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)



-
сигнальный язык
опасность
-
вредная бумага
H317:При контакте с кожей может вызывать аллергическую реакцию.
H411:Токсично для водных организмов с долгосрочными последствиями.
H225:Легковоспламеняющаяся жидкость. Пары образуют с воздухом взрывоопасные смеси.
-
оператор предупредительных мер
P210:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P233:Держать в плотно закрытой/герметичной таре.
P240:Заземлить и электрически соединить контейнер и приемное оборудование.
P273:Избегать попадания в окружающую среду.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P303+P361+P353:ПРИ ПОПАДАНИИ НА КОЖУ (или волосы): Снять/удалить немедленно всю загрязненную одежду. Промыть кожу водой.
2-метил-2-пропантиол химические свойства, назначение, производство
Описание
tert-Butylthiol, also known as 2-methyl propane-2-thiol, 2- methyl-2-propane thiol, tert-butyl mercaptan (TBM), and t-BuSH, is an organo sulfur compound with the formula (CH3)3CSH. This thiol may have been used as a flavoring agent, as an odorant for natural gas (which is odorless), and also in a wide range of organic reactions.Химические свойства
liquid with an exceedingly unpleasant smellИспользование
2-Methyl-2-propanethiol was used in reaction of 2-methyl-2-propanethiol on Mo(110) using temperature programmed reaction, high resolution electron enegy loss and X-ray photoelectron spectroscopies. It was used in the synthesis of chain-transfer agents for reversible addition-fragmentation chain-transfer copolymerization of vinylidene chloride and methyl acrylate.Подготовка
tert-Butyl thiol likely does not occur naturally, but at least one publication has listed it as a very minor component of cooked potatoes. The compound was first prepared in 1890 by Leonard Dobbin by the reaction of zinc sulfide and t-butyl chloride.The compound was later prepared in 1932 by the reaction of the Grignard reagent, t-BuMgCl, with sulfur to give the corresponding thiolate, followed by hydrolysis. This preparation is shown below:
t-BuMgCl + S → t-BuSMgCl
t-BuSMgCl + H2O → t-BuSH + Mg(OH)Cl
It is currently prepared industrially by the reaction of isobutylene with hydrogen sulfide over a clay (silica alumina) catalyst.
Реакции
tert-Butylthiol can react with metal alkoxides and acyl chlorides to form thiol esters, as shown in the equation :In the reaction above, thallium (I) ethoxide converts to thallium (I) t-butyl thiolate. In the presence of diethyl ether, thallium (I) tbutylthiolate reacts with acyl chlorides to give the corresponding tertbutyl thioesters. Like other thio esters, it reverts back to tert-butylthiol by hydrolysis.
Lithium 2-methyl propane-2-thiolate can be prepared by treatment of tert-butyl thiol with lithium hydride in an aprotic solvent such as hexa methyl phosphorous triamide (HMPT). The resulting thiolate salt is a useful demethylating reagent. For example, treatment with 7- methyl guanosine gives guanosine. Other N-methylated nucleosides in tRNA are not demethylated by this reagent .
Общее описание
2-Methyl-2-propanethiol undergoes ring opening nucleophilic reaction with 3-isothiazolones and reaction kinetics studies suggested reaction was second order in thiol and third order overall.Опасность
Flammable, dangerous fire risk. Very toxic.Профиль безопасности
Moderately toxic by intraperitoneal route. Mildly toxic by ingestion. An eye irritant. A very dangerous fire hazard when exposed to heat or flame. Can react vigorously with oxidizing materials. To fight fire, use alcohol foam, dry chemical, mist, fog. When heated to decomposition or on contact with acid or acid fumes it emits highly toxic fumes of SOx.Безопасность
Even in well ventilated areas, extreme caution must be made when handling tert-butylthiol as it is a highly odorous chemical with an odor threshold of < 0.33 ppb. Extreme caution is not due to toxicity, but due to the significant odor and concerns that this odor would cause to the many individuals that might be exposed. The PEL for thiols of most types is 500 ppb, primarily due to reaction of nausea at levels of 2–3 ppm. The LC50 of tert-butylthiol is much, much higher.Методы очистки
Dry the thiol for several days over CaO, then distil it from CaO. Purify it as for 2-methylpropane-1-thiol above. [Beilstein 1 H 383, 1 II 416, 1 III 1589, 1 IV 1634.]2-метил-2-пропантиол запасные части и сырье
2-метил-2-пропантиол поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | |
| +8617531153977 | China | 5855 | 58 | |
| +86-13131129325 | China | 5887 | 58 | |
| +86-(0)57185586718 +86-13336195806 |
China | 29792 | 60 | |
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| 0086-577-64498589 | CHINA | 998 | 58 | |
| 18871490254 | CHINA | 28172 | 58 | |
| 86-13657291602 | CHINA | 22963 | 58 | |
| +86-023-6139-8061 +86-86-13650506873 |
China | 39894 | 58 | |
| +8618523575427 | China | 49732 | 58 |
2-метил-2-пропантиол Обзор)
1of4