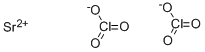
STRONTIUM CHLORATE
- русский язык имя
- английское имяSTRONTIUM CHLORATE
- CAS №7791-10-8
- CBNumberCB9429053
- ФормулаCl2O6Sr
- мольный вес254.52
- EINECS232-239-3
- номер MDLMFCD00054051
- файл Mol7791-10-8.mol
химическое свойство
| Температура плавления | 120° with decomposition and evolution of O2 |
| плотность | 3.15 |
| растворимость | slightly soluble in ethanol |
| цвет | colorless crystals, crystalline |
| Растворимость в воде | g/100g solution H2O: 61.40 (0°C), 63.78 (25°C), 67.08 (95°C); solid phase, Sr(ClO3)2 ·3H2O (0°C), Sr(ClO3)2 (25°C, 95°C) [KRU93]; slightly soluble alcohol [MER06] |
| FDA UNII | XG48A4P4FB |
| Система регистрации веществ EPA | Chloric acid, strontium salt (2:1) (7791-10-8) |
| РИДАДР | 1506 |
| Класс опасности | 5.1 |
| Группа упаковки | II |
| Банк данных об опасных веществах | 7791-10-8(Hazardous Substances Data) |
STRONTIUM CHLORATE химические свойства, назначение, производство
Описание
Strontium chlorate, Sr(ClO3)2, has a molecular weight of 254.52 g/mol. It is a white solid with a melting point of 120°C and a density of 3.152 g/cm3. It is soluble in water (174 g/100 ml at 20°C). Its CAS number is 7791-10-8. Sr(ClO3)2 is shock sensitive, and is highly flammable if allowed to sit in air. These white, water-soluble crystals decompose at 120°C. It is used in pyrotechnics and tracer bullets. There are two coexisting crystal hydrates that undergo interconversion, the dihydrate and the hexahydrate. These compounds are not as shock sensitive as the anhydrate.Химические свойства
White, crystalline powder. Soluble in water; slightly soluble in alcohol.Физические свойства
Strontium chlorate is a strong oxidizing agent. It forms a flammable mixture with combustible materials (can be ignited by friction). Such mixtures may be explosive.Contact with concentrated sulfuric acid can cause fires or explosions. When mixed with ammonium salts, spontaneous decomposition and ignition may result. This compound liberates explosive chlorine dioxide gas in the presence of strong acid; heating with a dibasic organic acid in the presence of moisture liberates chlorine dioxide and carbon dioxide. Mixtures with ammonium salts, powdered metals, silicon, sulfur, or sulfides are readily ignited and potentially explosive.
Использование
In pyrotechnics to produce red fire.Подготовка
Strontium chlorate can be produced by the Liebig method which consists of passing chlorine gas through a solid such as Sr(OH)2:6Sr(OH)2+ 6Cl2→5SrCl2+ Sr(CIO3)2+ H2O.
However, separating the two salts remains problematic since both are soluble in water. Strontium chloride solubility is 42.9 g/100 ml of water at 20°C while that of strontium chlorate is 174.9 g/100 ml of water at 20°C.
Общее описание
A moist solid or semi-solid slurry of white crystals. May explode under exposure to heat or fire. Used in pyrotechnics.Реакции воздуха и воды
Water soluble.Профиль реактивности
STRONTIUM CHLORATE is a strong oxidizing agent. Forms a flammable mixture with combustible materials (can be ignited by friction). Such mixtures may be explosive Contact with concentrated sulfuric acid can cause fires or explosions. When mixed with ammonium salts, spontaneous decomposition and ignition may result. Liberates explosive chlorine dioxide gas in the presence of strong acid; heating with a dibasic organic acid in the presence of moisture liberates chlorine dioxide and carbon dioxide [Bretherick 1979 p. 100]. Mixtures with ammonium salts, powdered metals, silicon, sulfur, or sulfides are readily ignited and potentially explosive [Bretherick 1979 p. 806].Опасность
Dangerous explosion risk in contact with organic materials; highly sensitive to shock, heat, and friction; strong oxidizing agent.Угроза здоровью
TOXIC; inhalation, ingestion or contact (skin, eyes) with vapors, dusts or substance may cause severe injury, burns or death. Fire may produce irritating and/or toxic gases. Toxic fumes or dust may accumulate in confined areas (basement, tanks, hopper/tank cars, etc.). Runoff from fire control or dilution water may cause pollution.Пожароопасность
May explode from friction, heat or contamination. These substances will accelerate burning when involved in a fire. May ignite combustibles (wood, paper, oil, clothing, etc.). Some will react explosively with hydrocarbons (fuels). Containers may explode when heated. Runoff may create fire or explosion hazard.Профиль безопасности
A powerful oxidizer. When heated to decomposition it emits toxic fumes of Cl-. See also CHLORATES.STRONTIUM CHLORATE запасные части и сырье
сырьё
STRONTIUM CHLORATE поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| 18871490254 | CHINA | 28172 | 58 | |
| 86-13657291602 | CHINA | 22963 | 58 | |
| +86-023-6139-8061 +86-86-13650506873 |
China | 39894 | 58 | |
| +86-0551-65418671 +8618949823763 |
China | 34563 | 58 | |
| +8615604608665 15604608665 |
CHINA | 9414 | 58 | |
| +8618950047208 | China | 43416 | 58 | |
| +86-0592-6210733 | China | 32343 | 55 | |
| 400-1166-196 13458535857 |
China | 13350 | 58 | |
| 4009903999 13395398332 |
China | 20806 | 60 | |
| 029-029-81148696 18161915376 |
China | 9997 | 58 |