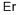
эрбий
- английское имяERBIUM
- CAS №7440-52-0
- CBNumberCB9375990
- ФормулаEr
- мольный вес167.26
- EINECS231-160-1
- номер MDLMFCD00010987
- файл Mol7440-52-0.mol
химическое свойство
| Температура плавления | 1529 °C (lit.) |
| Температура кипения | 2868 °C (lit.) |
| плотность | 9.062 g/mL at 25 °C (lit.) |
| показатель преломления | 1.47 (1300 nm) |
| температура хранения | 15-25°C |
| растворимость | insoluble in H2O; soluble in acid solutions |
| форма | powder |
| цвет | Silver-gray |
| Удельный вес | 9.006 |
| РН | 0.5 (20°C in H2O) |
| удельное сопротивление | 86 μΩ-cm, 20°C |
| Растворимость в воде | Soluble in acids. Insoluble in water. |
| Чувствительный | Moisture Sensitive |
| Мерк | 13,3675 |
| Пределы воздействия | ACGIH: TWA 2 ppm; STEL 4 ppm OSHA: TWA 2 ppm(5 mg/m3) NIOSH: IDLH 25 ppm; TWA 2 ppm(5 mg/m3); STEL 4 ppm(10 mg/m3) |
| Справочник по базе данных CAS | 7440-52-0(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | 77B218D3YE |
| Система регистрации веществ EPA | Erbium (7440-52-0) |
| UNSPSC Code | 41116107 |
| NACRES | NA.24 |
больше
| Коды опасности | F | |||||||||
| Заявления о рисках | 11 | |||||||||
| Заявления о безопасности | 43-Neverusewater. | |||||||||
| РИДАДР | UN 3089 4.1/PG 2 | |||||||||
| WGK Германия | 3 | |||||||||
| F | 1-10 | |||||||||
| TSCA | Yes | |||||||||
| Класс опасности | 8 | |||||||||
| Группа упаковки | III | |||||||||
| кода HS | 2846902035 | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
опасность
-
вредная бумага
H228:Воспламеняющееся твердое вещество.
-
оператор предупредительных мер
P210:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P240:Заземлить и электрически соединить контейнер и приемное оборудование.
P241:Использовать взрывобезопасное оборудование и освещение.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P370+P378:При пожаре тушить сухим песком, сухим химическим порошком или спиртостойкой пеной.
эрбий химические свойства, назначение, производство
Химические свойства
grey powderФизические свойства
Erbium is a soft, malleable metal with a silvery metallic luster that only tarnishes (oxidizes)slightly in air. It is one of the rare-earths of the yttrium subgroup of the lanthanide series.Its melting point is 1,529°C, its boiling point is 2,868°C, and its density is 9.07g/cm3.
Изотопы
There are 39 isotopes of erbium, six of which are stable: Er-162, Er-164, Er-166,Er-167, Er-168, and Er-170. These six isotopes make up the total atomic weight (mass)of erbium, and all the other isotopes are artificially made and short-lived. Their half-livesrange from 200 nanoseconds to 49 hours.Происхождение имени
Named for the quarry in Ytterby, Sweden, where ores and minerals of many elements are found.Вхождение
Erbium ranks 17th in abundance among the rare-earths, and it is the 46th most abundantelement found in the Earth’s crust. It exists in only 2.5 ppm, meaning that about 2.5 poundsof erbium could be extracted from one million pounds of dirt in the Earth’s crust. Higher concentrationsare found in some areas, but in general, the oxides of erbium are rather scarce.It is found in ores such as monazite, gadolinite, and bastnasite. It was first separated intothree elements in 1843 (yttria, erbia, and terbia). Erbium is also produced as a by-product ofnuclear fission of uranium.История
Erbium, one of the so-called rare-earth elements of the lanthanide series, is found in the minerals mentioned under dysprosium above. In 1842 Mosander separated “yttria,” found in the mineral gadolinite, into three fractions which he called yttria, erbia, and terbia. The names erbia and terbia became confused in this early period. After 1860, Mosander’s terbia was known as erbia, and after 1877, the earlier known erbia became terbia. The erbia of this period was later shown to consist of five oxides, now known as erbia, scandia, holmia, thulia and ytterbia. By 1905 Urbain and James independently succeeded in isolating fairly pure Er2O3. Klemm and Bommer first produced reasonably pure erbium metal in 1934 by reducing the anhydrous chloride with potassium vapor. The pure metal is soft and malleable and has a bright, silvery, metallic luster. As with other rare-earth metals, its properties depend to a certain extent on the impurities present. The metal is fairly stable in air and does not oxidize as rapidly as some of the other rare-earth metals. Naturally occurring erbium is a mixture of six isotopes, all of which are stable. Twenty-seven radioactive isotopes of erbium are also recognized. Recent production techniques, using ion-exchange reactions, have resulted in much lower prices of the rare-earth metals and their compounds in recent years. The cost of 99.9% erbium metal is about $21/g. Erbium is finding nuclear and metallurgical uses. Added to vanadium, for example, erbium lowers the hardness and improves workability. Most of the rare-earth oxides have sharp absorption bands in the visible, ultraviolet, and near infrared. This property, associated with the electronic structure, gives beautiful pastel colors to many of the rare-earth salts. Erbium oxide gives a pink color and has been used as a colorant in glasses and porcelain enamel glazes.Характеристики
Although erbium is magnetic at very low temperatures, it is antiferromagnetic and becomesa superconductor at temperatures near absolute zero. It is insoluble in water but soluble inacids. Its salts range from pink to red. Erbium and some of the other rare-earth elements areconsidered to be “impurities” in the minerals in which they are found. Small quantities oferbium can also be separated from several other rare-earths.Использование
Erbium has application in glass coloring, as an amplifier in fiber optics, and in lasers for medical and dental use.It is commonly used as a photographic filter, and because of its resilience it is useful as a metallurgical additive.
The Erbium ion has a very narrow absorption band coloring erbium salts pink. It is therefore used in eyeware and decorative glassware. It can neutralize discoloring impurities such as ferric ions and produce a neutral gray shade. It is used in a variety of glass products for this purpose.
Lasers based on Er:YAG are ideally suited for surgical applications because of its ability to deliver energy without thermal build-up in tissue.
Erbium Metal, is mainly metallurgical uses. Added to vanadium, for example, Erbium lowers hardness and improves workability. There are also a few applications for nuclear industry. Erbium Metal can be further processed to various shapes of ingots, pieces, wires, foils, slabs, rods, discs and powder.
Определение
A soft malleable silvery element of the lanthanoid series of metals. It occurs in association with other lanthanoids. Erbium has uses in the metallurgical and nuclear industries and in making glass for absorbing infrared radiation. Symbol: Er; m.p. 1529°C; b.p. 2863°C; r.d. 9.066 (25°C); p.n. 68; r.a.m. 167.26.Опасность
Erbium nitrate [Er(NO3)3] may explode when “shocked” or at high temperatures. As withother rare-earths, erbium and its compounds should be handled with care because they canbe toxic.эрбий запасные части и сырье
сырьё
эрбий поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86-18400010335 +86-18034520335 |
China | 1015 | 58 | |
| +86-18633929156 +86-18633929156 |
China | 972 | 58 | |
| +86-18532138899 +86-18532138899 |
China | 939 | 58 | |
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 | |
| +86 18953170293 | China | 2930 | 58 | |
| 18871490254 | CHINA | 28172 | 58 | |
| +86-023-6139-8061 +86-86-13650506873 |
China | 39894 | 58 | |
| 18503026267 | CHINA | 9636 | 58 | |
| +86-0551-65418671 +8618949823763 |
China | 34563 | 58 | |
| +86-029-89586680 +86-18192503167 |
China | 7724 | 58 |