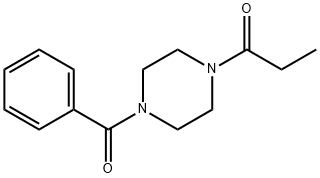
Пиперазин, 1-бензоил-4- (1-оксопропил) -
- английское имяSunifiram
- CAS №314728-85-3
- CBNumberCB92570478
- ФормулаC14H18N2O2
- мольный вес246.3
- EINECS1312995-182-4
- номер MDLMFCD04112755
- файл Mol314728-85-3.mol
химическое свойство
| Температура плавления | 92 - 94°C |
| Температура кипения | 442.0±38.0 °C(Predicted) |
| плотность | 1.154±0.06 g/cm3 (20 ºC 760 Torr) |
| температура хранения | 2-8°C |
| растворимость | H2O: soluble24mg/mL |
| пка | 0.08±0.70(Predicted) |
| форма | solid |
| цвет | white |
| InChI | InChI=1S/C14H18N2O2/c1-2-13(17)15-8-10-16(11-9-15)14(18)12-6-4-3-5-7-12/h3-7H,2,8-11H2,1H3 |
| ИнЧИКей | DGOWDUFJCINDGI-UHFFFAOYSA-N |
| SMILES | C(N1CCN(C(=O)C2=CC=CC=C2)CC1)(=O)CC |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | 66924E735K |
Пиперазин, 1-бензоил-4- (1-оксопропил) - химические свойства, назначение, производство
Описание
Sunifiram (DM235, CAS Number: 314728-85-3) is a novel pyrrolidone nootropic drug that was developed by the Gualtieri research group in 2000. It has a similar structure to piracetam, but it is not classified as a racetam drug due to the breaking of its pyrrolidone backbone. Sunifiram was originally developed to treat neurodegenerative disorders, such as Alzheimer's disease. Compared to piracetam, its anti-amnesiac activity is several orders of magnitude higher on a per-weight basis. The drugs can be helpful in the treatment of neurodegenerative disorders like Alzheimer’s, Parkinson’s, multiple sclerosis, schizophrenia, and attention-deficit hyperactivity disorders.Использование
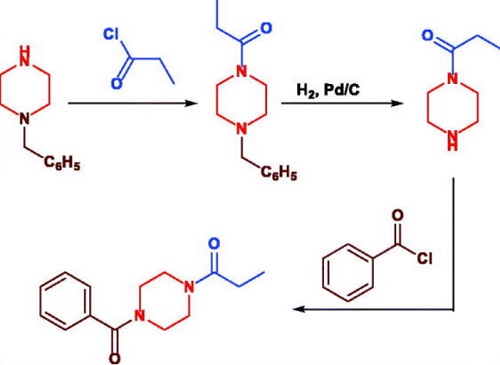
Sunifiram is a potent nootropic agent that provides a range of cognitive benefits. Its ability to improve concentration, focus, and mental clarity can greatly aid in the management and completion of cognitive tasks. In addition, it has been shown to enhance motivation, which is essential for achieving cognitive goals.Ссылки на синтез
Design, synthesis and nootropic activity of new analogues of sunifiram and sapunifiram, two potent cognition-enhancers.DOI: 10.1016/j.bmc.2009.08.055
Novel Sunifiram-carbamate hybrids as potential dual acetylcholinesterase inhibitor and NMDAR co-agonist: simulation-guided analogue design and pharmacological screening
DOI: 10.1080/14756366.2022.2068147
Побочные эффекты
While initial research suggests that sunifiram is virtually non-toxic and doesn’t produce any notable side effects, it’s important to note how little research has been conducted on this compound. For this reason, it’s incredibly important that you begin with a low dosage before ramping up to a higher one, and pay close attention to any noted side effects you experience.Based on anecdotal evidence, some sunifiram users have experienced side effects such as:
Headache
Hot flashes
Difficulty sleeping
Manic feelings
These side effects seem only to be mentioned with dosages outside of the 5-10mg range, with higher doses producing more pronounced side effects. If you find that you’re experiencing side effects as you up your dosage, ramp down your dosage to a level where no side effects are present.
Способ действия
Sunifiram enhances NMDA-dependent signaling by increasing PKCα phosphorylation, at a dosage range of 10-100nM. This mechanism of action is dependent on the availability of the glycine binding site and Sunifiram has been shown to act as an antagonist to glycine at a concentration of 300μM.Furthermore, the increased activity of AMPA receptor activation has been associated with increased CAMKII and PKCα phosphorylation. Although studies have confirmed that intracellular proteins like CAMKII and PKCα are activated after Sunifiram administration, other proteins like CaMKIV and ERK remain unaffected.
A brief summary of the mechanism of action, as it is currently understood, is that Sunifiram acts on the NMDA receptor’s glycine binding site, which results in increased signaling and activation of CAMKII and PKCα proteins and the subsequent positive regulation of AMPA receptors.
Furthermore, animal studies have indicated that a dosage of 0.01mg/kg increases acetylcholine release by up to 200% within one hour of injection, in rat prefrontal cortex. This effect is not seen at a higher dosage of 1mg/kg.
использованная литература
[1] Agha, Khalid A. et al. "Novel Sunifiram-carbamate hybrids as potential dual acetylcholinesterase inhibitor and NMDAR co-agonist: simulation-guided analogue design and pharmacological screening." Journal of Enzyme Inhibition and Medicinal Chemistry 37 (2022): 1241–1256. DOI: 10.1080/14756366.2022.2068147[2] Moriguchi, Shigeki et al. "Novel nootropic drug sunifiram enhances hippocampal synaptic efficacy via glycine‐binding site of N‐methyl‐D‐aspartate receptor." Hippocampus 23 (2013). DOI: 10.1002/hipo.22150
Пиперазин, 1-бензоил-4- (1-оксопропил) - поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +undefined-27-86652399 +undefined13627115097 |
China | 942 | 58 | ||
| +86-29-81148696 +86-15536356810 |
China | 3882 | 58 | ||
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | ||
| +86-16632316109 | China | 1085 | 58 | ||
| +8617756083858 | China | 973 | 58 | ||
| +1-027-87896938 +15172028168 |
China | 24 | 58 | ||
| +86-0086-57187702781 +8613675893055 |
China | 295 | 58 | ||
| +8613288715578 | China | 1174 | 58 | ||
| +8613363081709 | China | 300 | 58 | ||
| +86-16264648883 +86-16264648883 |
China | 3712 | 58 |