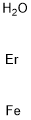
карбид циркония
- английское имяzirconium carbide
- CAS №12020-14-3
- CBNumberCB92131295
- ФормулаErFeH2O
- мольный вес241.12
- файл Mol12020-14-3.mol
химическое свойство
| Температура плавления | 3532°C |
| Температура кипения | 5100℃ [STR93] |
| плотность | 6.730 |
| растворимость | soluble in HF |
| форма | gray refractory solid |
| цвет | Gray |
| удельное сопротивление | 68 (ρ/μΩ.cm) |
| Растворимость в воде | soluble HF solutions which contain NO3? or peroxide [KIR84] |
| Кристальная структура | Cubic |
карбид циркония химические свойства, назначение, производство
Химические свойства
Zirconium carbide , nominally ZrC, is a dark gray brittle solid. It is made typically by a carbothermic reduction of zirconium oxide in a induction-heated vacuum furnace. Alternative production methods, especially for deposition on a substrate, consist of vapor-phase reaction of a volatile zirconium halide, usually ZrCl4, with a hydrocarbon in a hydrogen atmosphere at 900–1400 °C (1652–2552 °F).Физические свойства
Gray metallic solid; cubic structure; very hard, hardness > 8.0 Mohs; density 6.73 g/cm3; melts at 3,532°C; insoluble in water; slightly soluble in concentrated sulfuric acid; soluble in hydrofluoric acid and oxidizing acids, such as nitric and perchloric acids; attacked by oxidizers.Физические свойства
Dark gray brittle solid, soluble in HF solutions containing nitrate or peroxide ions. UC-nuclear power reactor, crucible container for handling molten metals such as Bi, Cd, Pb, Sn, Rb, and molten zirconia ZrO2. Corroded by liquid metals Mg, Al, Si, V, Nb, Ta, Cr, Mo, Mn, Fe, Co, Ni, and Zn. In air oxidizes rapidly above 500°C. Maximum operating temperature of 2350°C in helium.Использование
Zirconium carbide is a refractory material. It is used in making incandescent filaments, high temperature electrical conductors, and cutting tool components.Подготовка
Zirconium carbide is prepared by heating a mixture of zirconium oxide and coke in an arc furnace.карбид циркония запасные части и сырье
запасной предмет