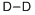
DEUTERIUM
- русский язык имя
- английское имяDEUTERIUM
- CAS №7782-39-0
- CBNumberCB8495776
- ФормулаD2
- мольный вес4.03
- EINECS231-952-7
- номер MDLMFCD00064812
- файл Mol7782-39-0.mol
химическое свойство
| Температура плавления | -254.43° (18.73 K) at 128.5 mm (triple point) |
| Температура кипения | -249.5 °C(lit.) |
| плотность | 0.169 g/mL at 25 °C(lit.) |
| плотность пара | 0.07 (vs air) |
| давление пара | 17.1-1665kPa at -254.43--234.8℃ |
| форма | gas |
| цвет | colorless |
| Мерк | 13,2956 |
| Диэлектрическая постоянная | 1.3(20℃) |
| Стабильность | Stable. Extremely flammable. Readily forms explosive mixtures with air. |
| Справочник по базе данных CAS | 7782-39-0(CAS DataBase Reference) |
| FDA UNII | AR09D82C7G |
| Система регистрации веществ EPA | Deuterium (7782-39-0) |
| Коды опасности | F+ | |||||||||
| Заявления о рисках | 12 | |||||||||
| Заявления о безопасности | 9-16-33 | |||||||||
| РИДАДР | UN 1957 2.1 | |||||||||
| WGK Германия | 1 | |||||||||
| Класс опасности | 2.1 | |||||||||
| Банк данных об опасных веществах | 7782-39-0(Hazardous Substances Data) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
опасность
-
вредная бумага
H220:Чрезвычайно легковоспламеняющийся газ.
H280:Газ под давлением. Баллоны (емкости) могут взрываться при нагревании.
-
оператор предупредительных мер
P210:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P410+P403:Беречь от солнечных лучей. Хранить в хорошо вентилируемом месте.
DEUTERIUM химические свойства, назначение, производство
Химические свойства
colourless gasИспользование
Used extensively in small amounts as tracer in the establishment of rates and kinetics of chemical reactions.Определение
A naturally occurring stable isotope of hydrogen in which the nucleus contains one proton and one neutron. The atomic mass is thus approximately twice that of 1H; deuterium is known as ‘heavy hydrogen’. Chemically it behaves almost identically to hydrogen, forming analogous compounds, although reactions of deuterium compounds are often slower than those of the corresponding 1H compounds. This is made use of in kinetic studies where the rate of a reaction may depend on transfer of a hydrogen atom (i.e. a kinetic isotope effect).Общее описание
DEUTERIUM is an isotope of hydrogen but DEUTERIUM is chemically identical. DEUTERIUM is a colorless, odorless gas. DEUTERIUM is easily ignited. Once ignited DEUTERIUM burns with a pale blue, almost invisible flame. The vapors are lighter than air. DEUTERIUM is flammable over a wide range of vapor/air concentrations. Under prolonged exposure to fire or intense heat the containers may rupture violently and rocket. DEUTERIUM is not toxic but is a simple asphyxiate by the displacement of oxygen in the air.Реакции воздуха и воды
Highly flammable.Профиль реактивности
DEUTERIUM, like hydrogen, is a reducing agent; reacts readily with oxidizing agents.Угроза здоровью
Vapors may cause dizziness or asphyxiation without warning. Some may be irritating if inhaled at high concentrations. Contact with gas or liquefied gas may cause burns, severe injury and/or frostbite. Fire may produce irritating and/or toxic gases.Пожароопасность
EXTREMELY FLAMMABLE. Will be easily ignited by heat, sparks or flames. Will form explosive mixtures with air. Vapors from liquefied gas are initially heavier than air and spread along ground. CAUTION: Hydrogen (UN1049), DEUTERIUM (UN1957), Hydrogen, refrigerated liquid (UN1966) and Methane (UN1971) are lighter than air and will rise. Hydrogen and DEUTERIUM fires are difficult to detect since they burn with an invisible flame. Use an alternate method of detection (thermal camera, broom handle, etc.) Vapors may travel to source of ignition and flash back. Cylinders exposed to fire may vent and release flammable gas through pressure relief devices. Containers may explode when heated. Ruptured cylinders may rocket.Сельскохозяйственное использование
Deuterium is one of the three isotopes of hydrogen, the other two being hydrogen-1 and tritium. Hydrogen-1 and deuterium are naturally occurring stable isotopes, while the radioactive tritium is made artificially.In nature, the ratio is one part of deuterium to 6500parts of normal hydrogen. Deuterium is present in water as the oxide HDO from which deuterium is usually obtained by electrolysis or fractional distillation. Its chemical behavior is similar to that of hydrogen, although deuterium compounds react slowly.
Синтез
Deuterium can be made by reacting D2O with metallic elements (Na, Mg and U). The reaction equation is shown below: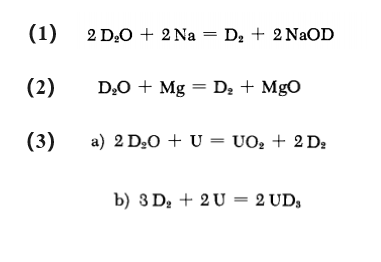
Методы очистки
Pass the gas over activated charcoal at -195o [MacIver & Tobin J Phys Chem 64 451 1960]. Purify it also by diffusion through nickel [Pratt & Rogers, J Chem Soc, Faraday Trans I 92 1589 1976]. Always check deuterium for radioactivity to determine the amount of tritium in it (see D2O below).DEUTERIUM запасные части и сырье
запасной предмет
DEUTERIUM поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| 86-13657291602 | CHINA | 22963 | 58 | |
| +8618523575427 | China | 49732 | 58 | |
| +86-0371-86658258 +8613203830695 |
China | 29809 | 58 | |
| +86-029-89586680 +86-18192503167 |
China | 7724 | 58 | |
| +86-021-50456736 +8613761615711 |
China | 1028 | 58 | |
| +8618950047208 | China | 43416 | 58 | |
| +86-0592-6210733 | China | 32343 | 55 | |
| 18824865657 | China | 509 | 58 | |
| 027-87382885 17702779303 |
China | 311 | 58 | |
| 0510-051082803581 15903302207 |
China | 229 | 58 |