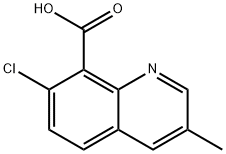
квинмерак
- английское имяQUINMERAC
- CAS №90717-03-6
- CBNumberCB8468273
- ФормулаC11H8ClNO2
- мольный вес221.64
- EINECS402-790-6
- номер MDLMFCD00145199
- файл Mol90717-03-6.mol
химическое свойство
| Температура плавления | 244°C |
| Температура кипения | 416.0±40.0 °C(Predicted) |
| плотность | 1.406±0.06 g/cm3(Predicted) |
| температура хранения | Room Temperature, under inert atmosphere |
| растворимость | DMSO (Slightly), Methanol (Slightly, Sonicated) |
| пка | -2.84±0.10(Predicted) |
| цвет | Pale Yellow to Pale Beige |
| Мерк | 13,8158 |
| БРН | 8392904 |
| LogP | 0.780 |
| FDA UNII | 0OFY83UPMH |
| Система регистрации веществ EPA | Quinmerac (90717-03-6) |
| UNSPSC Code | 41116107 |
| NACRES | NA.24 |
| Коды опасности | T |
| WGK Германия | 2 |
| RTECS | VB1981800 |
| кода HS | 29333990 |
| Токсичность | LD50 in rats (mg/kg): >5000 orally, >2000 dermally (Wuerzer) |
квинмерак химические свойства, назначение, производство
Химические свойства
Crystalline SolidОпределение
ChEBI: A quinolinemonocarboxylic acid that is quinoline-8-carboxylic acid carrying additional methyl and chloro substituents at positions 3 and 7 respectively. A residual herbicide used to control broad-leaved weeds on a range of crops including cereals, rape and beet.Экологическая судьба
In the field, the DT50 of quinmerac may range from 3 to 33 days. Losses due to volatilization are negligible. Soil moisture conditions greatly influence quinmerac persistence by moderating microbial degradation and soil leaching. Quinmerac is only slightly adsorbed to the soil.Метаболизм
Chemical. Quinmerac is stable to heat, light, and in aqueous solutions with pH values between 3 and 9.Plant. The degradation and metabolic pathways of quinmerac have not been extensively studied. In plants, oxidation of the 3-methyl group to the alcohol and hydroxylation at the 2-quinoline position are the major metabolism reactions (56). These quinmerac metabolites are subsequently conjugated to carbohydrates. The quantity of quinmerac metabolized varies among species, ranging from 5% to 80% (56).
Soil. In the soil, degradation of quinmerac was similar to that observed in plants, resulting in the same oxygenated and hydroxylated metabolites.
квинмерак запасные части и сырье
квинмерак поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| 18871490254 | CHINA | 28172 | 58 | |
| 86-13657291602 | CHINA | 22963 | 58 | |
| +8618523575427 | China | 49732 | 58 | |
| +86-0371-86658258 +8613203830695 |
China | 30233 | 58 | |
| +86-0551-65418684 +8618949823763 |
China | 25356 | 58 | |
| +86-0512-83500002 +8615195660023 |
China | 23046 | 58 | |
| +86-852-30606658 | China | 43340 | 58 | |
| +86-18192503167 +86-18192503167 |
China | 1022 | 58 | |
| +8618950047208 | China | 43416 | 58 | |
| 571-89925085 | China | 131957 | 58 |