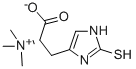
L-(+)-эрготионеин
- английское имяL-(+)-Ergothioneine
- CAS №497-30-3
- CBNumberCB8460398
- ФормулаC9H15N3O2S
- мольный вес229.3
- EINECS207-843-5
- номер MDLMFCD00167474
- файл Mol497-30-3.mol
химическое свойство
| Температура плавления | 275-277°C (dec.) |
| плотность | 1.2541 (rough estimate) |
| показатель преломления | 1.6740 (estimate) |
| температура хранения | -20°C |
| растворимость | Soluble in Water (up to 10 mg/ml) |
| форма | White solid. |
| пка | 1.706±0.32(predicted) |
| цвет | White or off-white |
| РН | +47^o (c=1 in water) |
| Биологические источники | fungus (Actinomycetales) fungus (Ascomycota) fungus (Basidiomycota) |
| Растворимость в воде | water: 50mg/mL, clear, colorless |
| Стабильность | Stable for 1 year from date of purchase as supplied. Solutions in water may be stored at -20°C for no more then 1 day. |
| InChI | InChI=1/C9H15N3O2S/c1-12(2,3)7(8(13)14)4-6-5-10-9(15)11-6/h5,7H,4H2,1-3H3,(H2-,10,11,13,14,15)/t7-/s3 |
| ИнЧИКей | SSISHJJTAXXQAX-KPOCXSGKNA-N |
| SMILES | [N+](C)(C)(C)[C@@H](CC1=CNC(S)=N1)C([O-])=O |&1:4,r| |
| FDA UNII | BDZ3DQM98W |
| Система регистрации веществ EPA | 1H-Imidazole-4-ethanaminium, .alpha.-carboxy-2,3-dihydro-N,N,N-trimethyl-2-thioxo-, inner salt, (.alpha.S)- (497-30-3) |
| UNSPSC Code | 12352209 |
| NACRES | NA.32 |
больше
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H315:При попадании на кожу вызывает раздражение.
H319:При попадании в глаза вызывает выраженное раздражение.
H335:Может вызывать раздражение верхних дыхательных путей.
-
оператор предупредительных мер
P280:Использовать перчатки/ средства защиты глаз/ лица.
P302+P352:ПРИ ПОПАДАНИИ НА КОЖУ: Промыть большим количеством воды.
P304+P340:ПРИ ВДЫХАНИИ: Свежий воздух, покой.
L-(+)-эрготионеин химические свойства, назначение, производство
Описание
L-Химические свойства
White SolidOrigin
Ergothioneine was discovered in 1909 by Charles Tanret, a French pharmacist and chemist. Tanret was examining the ergot fungus, which had recently been responsible for destroying crops, and he discovered the compound by using a purification process. The amino acid name ergothioneine originates from this fungus. Though this discovery is relatively recent, scientists speculate that ergothioneine may have originated from ancient earth. Due to its anaerobic nature (it does not require oxygen to function), it may have manifested in the earth's oxygen-free atmosphere more than three billion years ago While ergothioneine is not classified as one of the nine essential amino acids.преимущество
L-(+)-Ergothioneine is a natural antioxidant, which has various physiological functions such as scavenging free radicals, detoxification, maintaining DNA biosynthesis, normal cell growth and cellular immunity.Общее описание
L-(+)-Ergothioneine (ERG) is a substance that cannot be synthesized by humans and must be obtained from food. It has cytoprotective and antioxidant properties.Общее описание
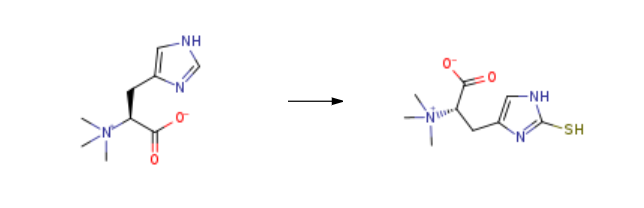
L-(+)-Ergothioneine (ET) is a sulfur-containing amino acid, which is only produced by Actinomycetales bacteria and non-yeast like fungi belonging to the division Basidiomycota and Ascomycota. It was originally isolated from Claviceps purpurea or rye ergot. It is obtained from L-histidine, which is converted into betaine form called hercynine. It is found in both animals and plants, and mammals usually obtain it from their diet, e.g. through mushrooms or oats. It is tautomeric in nature, and in neutral aqueous solution exists in thione form.Синтез
L-(+)-Ergothioneine is prepared by the reaction of hercynine. The steps are as follows:15g of compound was added to 150ml of water, and 15.6g of concentrated hydrochloric acid was added, add 10.9g dibromohydantoin, stir for 20min, add D-cysteine, Continue to stir for 1 hour, add sodium thiosulfate, raise the temperature to 90-100°C, and continue the reaction for 15 hours. After the reaction, cool down and filter, adjust the pH to neutral, desalt, concentrate, crystallize with 5ml of water and 75ml of isopropanol, and dissolve the solid Filter and dry at 70-80°C to obtain 88% ergothioneine product with a yield of 81%.
L-(+)-эрготионеин поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +86-0572 2526078; +8613757061525 |
China | 18 | 58 | ||
| +86-021-60410486 +8618621797362 |
China | 7 | 58 | ||
| 18283602253; +8618283602253 |
China | 953 | 58 | ||
| +86-75589664337 +86-15112661142 |
China | 58 | 58 | ||
| +86-18136843612 +86-19951791336 |
China | 1369 | 58 | ||
| +86-731-82791134 +86-17673622440 |
China | 30 | 58 | ||
| +8618058761490 | China | 49983 | 58 | ||
| +86-0571-58605786 +8617758008775 |
China | 47 | 58 | ||
| +8618782242822 | China | 8 | 58 | ||
| +8615531157085 | China | 8804 | 58 |