Этанерцепт
- английское имяEtanercept
- CAS №185243-69-0
- CBNumberCB81373136
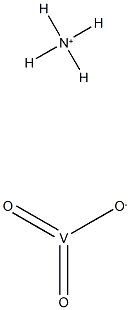
- ФормулаH4NO3V
- мольный вес116.97816
- номер MDLMFCD04605453
- файл Mol185243-69-0.mol
| температура хранения | Store at 4°C, Do not freeze |
| форма | Liquid |
| цвет | Colorless to light yellow |
| Растворимость в воде | Soluble in water |
| FDA UNII | OP401G7OJC |
| Словарь онкологических терминов NCI | etanercept |
| Словарь наркотиков NCI | Enbrel |
| Код УВД | L04AB01 |
| UNSPSC Code | 41116107 |
| NACRES | NA.24 |
| Банк данных об опасных веществах | 185243-69-0(Hazardous Substances Data) |
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H351:Предполагается, что данное вещество вызывает раковые заболевания.
-
оператор предупредительных мер
P201:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P202:Перед использованием ознакомиться с инструкциями по технике безопасности.
P281:Пользоваться надлежащим индивидуальным защитным снаряжением.
P308+P313:ПРИ подозрении на возможность воздействия обратиться за медицинской помощью.
P405:Хранить в недоступном для посторонних месте.
P501:Удалить содержимое/ контейнер на утвержденных станциях утилизации отходов.
Этанерцепт химические свойства, назначение, производство
Описание
Etanercept is produced by recombinant DNA technology in a Chinese hamster ovary mammalian cell line and is the first biotechnology-derived drug to be introduced for the reduction of the signs and symptoms of moderately to severely active rheumatoid arthritis in patients who have not adequately responded to one or more of the synthetic DMARDs. It is a dimeric soluble form of the p75 TNFR capable of binding to two TNF molecules in the circulation. It consists of the extracellular ligand binding portion of the 75-kDa human TNFR fused to the Fc portion of human IgG1.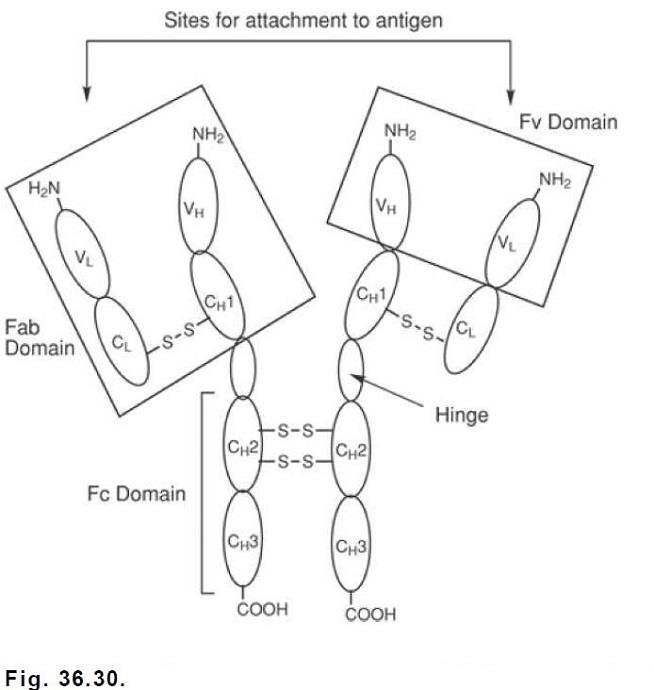
The Fc component of etanercept contains the CH2 domain, the CH3 domain, and the hinge region, but not the CH1 domain of IgG1. It consists of 934 amino acids and has an apparent molecular weight of approximately 150 kDa. Two TNFRs have been identified, a 75-kDa protein and a 55-kDa protein, that occur as monomeric molecules on cell surfaces and soluble forms in the blood. The biological activity of TNF requires its binding to either of the two cell surface TNFRs. Etanercept can bind specifically to two molecules of TNFα in the circulation, preventing its interaction with cell surface TNFRs.
Использование
To decrease signs and symptoms of rheumatoid arthritis.
Etanercept (Enbrel) is a soluble TNF receptor fusion protein. It is FDA approved for moderate to severe psoriasis at a dose of 50 mg twice weekly by subcutaneous injection for the initial 12 weeks, followed by a stepdown to 50 mg weekly for maintenance. The most common side effect is injection site reactions. Rare cases of serious infection, demyelinating disease, and congestive heart failure have been reported by postmarketing surveillance.
Показания
Etanercept (Enbrel) is a recombinant fusion protein designed to block the action of TNF-α. The drug is composed of the extracellular ligand-binding portion of the 75-kilodalton human TNF receptor linked to the Fc portion of human IgG1. TNF-α is a cytokine thought to play a major role in the pathogenesis of a number of inflammatory skin diseases, including psoriasis. Etanercept binds soluble TNF-α, preventing it from binding to and activating receptors for TNF that are present on cell membranes.Фармаколо?гия
Etanercept (Enbrel) is a soluble TNF receptor fusion protein. It is FDA approved for moderate to severe psoriasis at a dose of 50 mg twice weekly by subcutaneous injection for the initial 12 weeks, followed by a stepdown to 50 mg weekly for maintenance.Клиническое использование
Etanercept is approved in the United States for the treatment of psoriatic arthritis and rheumatoid arthritis. Although etanercept has not been specifically approved for the treatment of the cutaneous manifestations of psoriasis, it significantly improves the skin lesions of patients with moderate to severe cutaneous psoriasis who have used it for psoriatic joint disease.Побочные эффекты
The most common adverse reaction to etanercept is mild to moderate erythema, pain, or pruritus at the injection site (37%). Headaches and abdominal pain can also occur. New positive autoantibodies, such as antinuclear antibodies (ANA), anti-dsDNA antibodies, and anticardiolipin antibodies, can develop in patients treated with etanercept. Although there is so far no association between this and the development of autoimmune diseases or malignancies, long-term studies have yet to be done. Rare cases of pancytopenia may be associated with this drug. Although clinical trials showed no increased risk of infection with etanercept treatment, postmarketing reports of serious infections, sepsis, and associated fatalities exist.Меры предосторожности
Etanercept therapy should not be initiated in patientswith active infection. If an infection develops in a persontaking etanercept, he or she should be closely monitored.If a serious infection or sepsis occurs, the drugshould be discontinued. Etanercept should be usedwith caution in individuals who have conditions predisposingthem to serious infection (e.g., uncontrolleddiabetes, hematological abnormalities). Data on druginteractions are limited. Live virus vaccines are contraindicatedbecause of the potential for secondarytransmission of the infection by the vaccine. Myelosuppressiveantirheumatic agents have been associatedwith pancytopenia in some patients treated with etanercept.Этанерцепт поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +1-781-999-5354 +1-00000000000 |
United States | 32161 | 58 | |
| +86-29-89586680 +86-15129568250 |
China | 22787 | 58 | |
| +86-0571-85134551 | China | 15352 | 58 | |
| +8615056975894 | CHINA | 9911 | 58 | |
| 571-86758373 +8613588754946 |
CHINA | 3148 | 58 | |
| +8618058761490 | China | 49983 | 58 | |
| +8618327326525 | China | 8467 | 58 | |
| +86-18621343501; +undefined18621343501 |
China | 33338 | 58 | |
| +8613817748580 | China | 40066 | 58 | |
| 571-89925085 | China | 131957 | 58 |