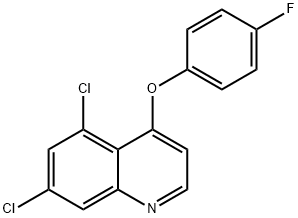
QUINOXYFEN
- русский язык имя
- английское имяQUINOXYFEN
- CAS №124495-18-7
- CBNumberCB7757516
- ФормулаC15H8Cl2FNO
- мольный вес308.13
- EINECS602-997-3
- номер MDLMFCD03265638
- файл Mol124495-18-7.mol
химическое свойство
| Температура плавления | 105-106° |
| Температура кипения | 423℃ |
| плотность | 1.430 |
| давление пара | 1.2 x 10-5 Pa (20 °C) |
| Fp | >100 °C |
| температура хранения | 0-6°C |
| растворимость | Chloroform (Slightly), Methanol (Slightly) |
| форма | Solid |
| Растворимость в воде | 0.12 mg l-1 (20 °C) |
| пка | 2.87±0.50(Predicted) |
| цвет | White to Light yellow |
| Мерк | 14,8079 |
| LogP | 5.1 at 20℃ |
| Dissociation constant | 3.56 |
| Рейтинг продуктов питания EWG | 1-2 |
| FDA UNII | PPC78J1VCW |
| Система регистрации веществ EPA | Quinoxyfen (124495-18-7) |
| UNSPSC Code | 41116107 |
| NACRES | NA.24 |
больше
| Коды опасности | Xi,N |
| Заявления о рисках | 43-50/53 |
| Заявления о безопасности | 24-37-46-60-61 |
| РИДАДР | UN3077 9/PG 3 |
| WGK Германия | 3 |
| RTECS | VB4287500 |
| Класс опасности | 9 |
| Группа упаковки | III |
| кода HS | 38220090 |
| Банк данных об опасных веществах | 124495-18-7(Hazardous Substances Data) |
| Токсичность | LD50 orally in rats: >5000 mg/kg; dermally in rabbits: >2000 mg/kg; by inhalation in rats: >3.38 mg/l (Longhurst) |
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
предупреждение
-
вредная бумага
H317:При контакте с кожей может вызывать аллергическую реакцию.
H410:Чрезвычайно токсично для водных организмов с долгосрочными последствиями.
-
оператор предупредительных мер
P261:Избегать вдыхания пыли/ дыма/ газа/ тумана/ паров/ аэрозолей.
P272:Не уносить загрязненную спецодежду с места работы.
P273:Избегать попадания в окружающую среду.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P302+P352:ПРИ ПОПАДАНИИ НА КОЖУ: Промыть большим количеством воды.
P333+P313:При возникновении раздражения или покраснения кожи обратиться за медицинской помощью.
QUINOXYFEN химические свойства, назначение, производство
Химические свойства
Off-White SolidИспользование
Quinoxyfen is under development for the control of powdery mildew in cereals and grapes.Определение
ChEBI: A member of the class of quinolines carrying two chloro substituents at positions 5 and 7 together with a 4-fluorophenoxy substituent at position 4. A fungicide used mainly to control powdery mildew in cereals.Экологическая судьба
The stability of quinoxyfen in the soil has been shown to vary depending on soil type and source. DT50 values obtained in the field varied from 5 to 454 days for a range of soil types. The strong adsorptive properties of quinoxyfen reduce its soil dissipation rate, but result in no leaching potential of this fungicide into waterways or groundwater. The primary metabolite formed in the soil is 3-hydroxyquinoxyfen . A secondary soil metabolite is 5,7-dichloro-4-hydroxyquinoline (DCHQ). DCHQ was also found not to leach, even in sandy soils. Under acidic aqueous conditions, DCHQ was the primary metabolite found, and this was produced in greater quantities at acidic pHs. An additional metabolite was isolated from both water and the sediment in an aqueous clay loam system. Although not positively identified, it is suspected to be 6-hydroxyquinoxyfen.A similar hydrolysis profile is observed in water as in soil (3). The primary product produced under acidic conditions in the absence of light was DCHQ. However, in the presence of light, photolysis was greatly increased and dose dependent on the amount of sunlight received. The primary photolysis product was 2-chloro-10- fluoro[1]benzopyrano[2,3,4-de]quinoline (CFBPQ).
Метаболический путь
Quinoxyfen is a novel fungicide for the control of powdery mildew in cereals. Its mode of action is unknown but it appears to differ from those of current fungicides and thus may be novel (Longhurst et al., 1996). Quinoxyfen is tightly bound to soil components and is somewhat persistent in this medium but in aqueous solution it is subject to rapid photodecomposition. Photodegradation is therefore likely to be an important process in its immediate removal from the environment. Plant metabolites have not been reported but unchanged quinoxyfen has been confirmed as the major residue.The compound is rapidly metabolised and eliminated following ingestion by rats and goats. Metabolism involves mainly hydroxylation of the intact quinoxyfen, cleavage at the ether bond and conjugation of the resulting metabolites. The information presented below was obtained from two sources (DowElanco, 1996; Reeves et al., 1996).
QUINOXYFEN запасные части и сырье
QUINOXYFEN поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +undefined-21-51877795 | China | 32965 | 60 | |
| +8618957127338 | China | 2136 | 58 | |
| 18905173768 | CHINA | 2972 | 58 | |
| 18871490254 | CHINA | 28172 | 58 | |
| 86-13657291602 | CHINA | 22963 | 58 | |
| +86-023-6139-8061 +86-86-13650506873 |
China | 39894 | 58 | |
| +86-13806087780 | China | 17365 | 58 | |
| +1-781-999-5354 +1-00000000000 |
United States | 32161 | 58 | |
| +86-0571-85134551 | China | 15352 | 58 | |
| +86-0551-65418684 +8618949823763 |
China | 25356 | 58 |