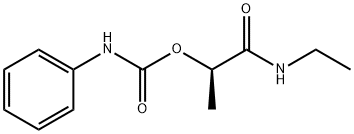
CARBETAMIDE
- русский язык имя
- английское имяCARBETAMIDE
- CAS №16118-49-3
- CBNumberCB7717695
- ФормулаC12H16N2O3
- мольный вес236.27
- EINECS240-286-6
- номер MDLMFCD00135093
- файл Mol16118-49-3.mol
| Температура плавления | >110°C |
| Температура кипения | 378.73°C (rough estimate) |
| плотность | 1.174 |
| показатель преломления | 1.5460 (estimate) |
| температура хранения | 0-6°C |
| растворимость | Chloroform (Slightly), Methanol (Slightly) |
| пка | 13.22±0.70(Predicted) |
| Растворимость в воде | 3.5g/L(20 ºC) |
| БРН | 8318291 |
| LogP | 1.520 (est) |
| FDA UNII | A75ON2B2V7 |
| Система регистрации веществ EPA | Carbetamide (16118-49-3) |
| UNSPSC Code | 41116107 |
| NACRES | NA.24 |
| Коды опасности | Xi |
| WGK Германия | 2 |
| RTECS | FD8900000 |
| кода HS | 29242990 |
| Токсичность | dog,LD50,oral,900mg/kg (900mg/kg),Guide to the Chemicals Used in Crop Protection. Vol. 6, Pg. 80, 1973. |
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)



-
сигнальный язык
опасность
-
вредная бумага
H302:Вредно при проглатывании.
H351:Предполагается, что данное вещество вызывает раковые заболевания.
H360D:Может отрицательно повлиять на неродившегося ребенка.
H411:Токсично для водных организмов с долгосрочными последствиями.
-
оператор предупредительных мер
P201:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P273:Избегать попадания в окружающую среду.
P301+P312+P330:ПРИ ПРОГЛАТЫВАНИИ: Обратиться за медицинской помощью при плохом самочувствии. Прополоскать рот.
P308+P313:ПРИ подозрении на возможность воздействия обратиться за медицинской помощью.
CARBETAMIDE химические свойства, назначение, производство
Химические свойства
Carbetamide is a colorless, crystalline powder, or solid.Определение
ChEBI: A carbamate ester obtained by the formal condensation of phenylcarbamic acid with the hydroxy group of N-ethyl-2-hydroxypropanamide.Возможный контакт
Carbamate herbicide used to kill unwanted plantsМетаболический путь
Photodegradation of carbetamide in solution with UV irradiation in the presence of UV ? H2O2 and ? TiO2 primarily occurs at the o- and p-positions, but not at the m-position of the phenyl ring, and the preferential photoproduct isolated is ortho-hydroxylated carbetamide. 5-Methyl-3-phenyl-oxazolidine dione is isolated only in the presence of TiO2. The cyclization may result from radical coupling with the dissolved oxygen. N-De-ethylation of the ethylcarbamoyl group and hydroxylation on the carbamoyloxy linkage occur to yield free amine of carbetamide and lactamide analogs. It is interesting to note that formulated carbetamide is phototransformed to N-ethyllactamide- 4-aminobenzoate as a major product via the rearrangement reaction similar to the Photo-Fries reaction. The degradation kinetics of carbetamide and its potential metabolites are measured at different temperatures in both unsterilized and sterilized alkaline soil.The chemical degradation of carbetamide importantly gives N-phenyl-3-methyloxazolidine-2,5- dione which results in 2-(phenylcarbamoyloxy)- propionic acid and N-phenyl-2-hydroxypropionamide and the aniline in the soil. The purely biological degradation of this compound cannot clearly yield degradation products that are different from those by chemical degradation in non-sterilized soils.
Перевозки
UN2757 Carbamate pesticides, solid, toxic, Hazard Class: 6.1; Labels: 6.1-Poisonous materials. UN 2811 Toxic solids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name RequiredНесовместимости
Slowly hydrolyzes in water, releasing ammonia and forming acetate salts. Carbamates are incompatible with reducing agents, strong acids, oxidizing acids, peroxides, and bases. Contact with active metals or nitrides cause the release of flammable, and potentially explosive, hydrogen gas. Amides react with azo and diazo compounds to generate toxic gases. Flammable gases are formed by the reaction of organic amides with strong reducing agents such as hydrideds and active metals. Amides are very weak bases (weaker than water). Mixing amides with dehydrating agents such as phosphorus pentoxide or thionyl chloride generates the corresponding nitrile. The combustion of these compounds generates mixed oxides of nitrogen (NOx). This compound is decomposed by strong base or acid.Утилизация отходов
Do not discharge into drains or sewers. Dispose of waste material as hazardous waste using a licensed disposal contractor to an approved land fill. Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Incineration with effluent gas scrubbing is recommended. Containers must be disposed of properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office.CARBETAMIDE запасные части и сырье
CARBETAMIDE поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| 18871490254 | CHINA | 28172 | 58 | |
| +86-023-6139-8061 +86-86-13650506873 |
China | 39894 | 58 | |
| 8485655694 | United States | 63687 | 58 | |
| +8618530059196 | China | 11805 | 58 | |
| +1-781-999-5354 +1-00000000000 |
United States | 32161 | 58 | |
| +86-0551-65418671 +8618949823763 |
China | 34563 | 58 | |
| 571-88938639 +8617705817739 |
China | 52849 | 58 | |
| +86-852-30606658 | China | 24727 | 58 | |
| +8618058761490 | China | 49983 | 58 | |
| +86-18621343501; +undefined18621343501 |
China | 33338 | 58 |