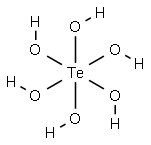
TELLURIC ACID
- русский язык имя
- английское имяTELLURIC ACID
- CAS №7803-68-1
- CBNumberCB7673964
- ФормулаH6O6Te
- мольный вес229.64404
- EINECS232-267-6
- номер MDLMFCD00011609
- файл Mol7803-68-1.mol
химическое свойство
| Температура плавления | 136°C |
| Температура кипения | 0℃ |
| плотность | 3.068; d 3.163 |
| растворимость | H2O: 0.1 g/mL, clear, colorless |
| пка | 7.7(at 25℃) |
| форма | white monoclinic crystals |
| цвет | White to Almost white |
| Растворимость в воде | g H2TeO6/100g H2O: 16.2 (0°C), 41.6 (20°C), 155 (100°C) [LAN05]; tends to polymerize; soluble dilute HNO3 [MER06] |
| λмакс | 275nm(H2O)(lit.) |
| Мерк | 14,9120 |
| Константа произведения растворимости (Ksp) | pKsp: 53.52 |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | WE1KZR49WU |
| Система регистрации веществ EPA | Telluric acid (H6TeO6) (7803-68-1) |
| Коды опасности | Xn |
| Заявления о рисках | 20 |
| Заявления о безопасности | 22-36/37/39-38 |
| РИДАДР | UN 3284 6.1/PG 3 |
| WGK Германия | 3 |
| RTECS | WY2350000 |
| F | 8 |
| кода HS | 2811.19.6190 |
| Класс опасности | 6.1(a) |
| Группа упаковки | II |
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H332:Вредно при вдыхании.
-
оператор предупредительных мер
P261:Избегать вдыхания пыли/ дыма/ газа/ тумана/ паров/ аэрозолей.
P271:Использовать только на открытом воздухе или в хорошо вентилируемом помещении.
P304+P340+P312:ПРИ ВДЫХАНИИ: Свежий воздух, покой. Обратиться за медицинской помощью при плохом самочувствии.
TELLURIC ACID химические свойства, назначение, производство
Описание
Telluric acid, H6TeO6, is obtained in the form of its salts when tellurium is fused with potassium nitrate plus carbonate, or by the oxidizing action of chlorine on a tellurite in alkaline solution. The free acid may be obtained by decomposing the barium salt with sulfuric acid and concentrating the solution.where tellurium is in an octahedral coordination. Telluric acid, Te(OH)6, has the CAS number of 7803-68- 1 and a molecular weight of 229.6425 g/mol. It is a white, monoclinic crystal with a density of 3.07 g/cm3. Its melting point is 136°C and its solubility in water is 50.1 g/100 ml.
It is also formed when tellurium dioxide is oxidized by hydrogen peroxide in caustic solution (A. Gutbier, Zest. Anorg. Chem., 1904, 40, p. 260), and perhaps best of all by oxidizing tellurium with a mixture of nitric and chromic acids. It crystallizes as prisms, which lose their water of hydration at 160°C. The tellurates of the alkaline earth metals are more or less soluble in water, those of the other metals being very sparingly or almost insoluble in water. Some tellurates exist in two forms, a colorless form soluble in water and acids, and a yellow form insoluble in water and acids.
Химические свойства
White, heavy crystals. Soluble in hot water and alkalies; slightly soluble in cold water.Физические свойства
White crystals; dimorphic solid; exists in both cubic and monoclinic crystalline forms; density 3.07g/cm3; melts at 136°C; tends to polymerize (similar to stannic acid); forms polymetatelluric acid (H2TeO4)n on strong heating; soluble in water, about 33g/100 mL at 30°C; the solubility decreases as the molecule polymerizes and becomes colloidal; a very weak dibasic acid, pKa1 7.68 and pKa2 11.0 at 18°C; soluble in dilute nitric acid and alkalies.Использование
Chemical reagent.Подготовка
Telluric acid can be prepared by reducing barium tellurate with sulfuric acid: BaTeO4 + H2SO4 + 2H2O → H6TeO6 + BaSO4 Also, telluric acid can be prepared by oxidation of tellurium or tellurium dioxide with a strong oxidizing agent such as hydrogen peroxide, sodium peroxide, chromic acid, or potassium permanganate in nitric acid. Molecular equations for overall reactions are shown below: Te + 3H2O2 → H6TeO6 TeO2 + H2O2 + 2H2O → H6TeO6 Te + 2CrO3 + 3H2O → H6TeO6 + Cr2O3At cold temperatures at about 1°C, telluric acid crystallizes as tetrahydrate.
Общее описание
Telluric acids exist in both cubic and monoclinic crystalline forms.1 It may be prepared by treating tellurium and tellurous acid with strong oxidants.Опасность
As for tellurium.Профиль безопасности
Poison by ingestion and intravenous routes. When heated to decomposition it emits toxic fumes of Te. See also TELLURIUM COMPOUNDS.Методы очистки
Crystallise it once from nitric acid, then repeatedly from hot water (0.4mL/g). [Fehér in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I pp 451-453 1963.]TELLURIC ACID запасные части и сырье
запасной предмет
TELLURIC ACID поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| 18871490254 | CHINA | 28180 | 58 | |
| 86-13657291602 | CHINA | 22968 | 58 | |
| +86-023-6139-8061 +86-86-13650506873 |
China | 39916 | 58 | |
| +86-0371-86658258 +8613203830695 |
China | 29825 | 58 | |
| 18503026267 | CHINA | 9641 | 58 | |
| +86 13288715578 +8613288715578 |
China | 12449 | 58 | |
| +86-0551-65418671 +8618949823763 |
China | 34571 | 58 | |
| +86-029-89586680 +86-18192503167 |
China | 8840 | 58 | |
| +8618339805032 | China | 13185 | 58 | |
| United States | 24072 | 58 |