CARBOXYLIC ACID
- русский язык имя
- английское имяCARBOXYLIC ACID
- CAS №
- CBNumberCB7526017
- ФормулаC13H9ClFNO3
- мольный вес281.67
- файл MolMol file
CARBOXYLIC ACID химические свойства, назначение, производство
Определение
A type of organic compound with the general formula RCOOH. Many carboxylic acids occur naturally in plants and (in the form of esters) in fats and oils, hence the alternative name fatty acids. Carboxylic acids with one COOH group are called monobasic, those with two, dibasic, and those with three, tribasic. The methods of preparation are:1. Oxidation of a primary alcohol or an aldehyde:
RCH2OH + 2[O] → RCOOH + H2O
2. Hydrolysis of a nitrile using dilute hydrochloric acid:
RCN + HCl + 2H2O → RCOOH + NH4Cl
The acidic properties of carboxylic acids are due to the carbonyl group, which attracts electrons from the C-O and O-H bonds. The carboxylate ion formed, R-COO–, is also stabilized by delocalization of electrons over the O-C-O grouping.
Other reactions of carboxylic acids include the formation of esters and the reaction with phosphorus(V) chloride to form acyl halides.
Реакции
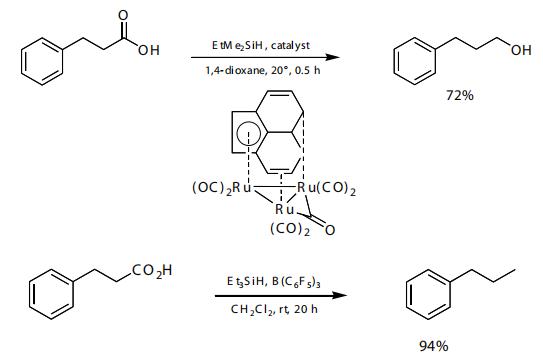
Silane Reduction of Carboxylic Acids is as follows:Ethyldimethylsilane and a ruthenium catalyst were used to reduce aliphatic carboxylic acids to the corresponding alcohol. With tris(pentaflfl uorophenyl)borane as catalyst, triethylsilane reduces carboxylic acids to the alkane.