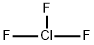
ХЛОРА ТРИФТОРИД
- английское имяCHLORINE TRIFLUORIDE
- CAS №7790-91-2
- CBNumberCB7437735
- ФормулаClF3
- мольный вес92.45
- EINECS232-230-4
- номер MDLMFCD00042534
- файл Mol7790-91-2.mol
| Температура плавления | -83°C |
| Температура кипения | 11,75°C |
| плотность | 1,8 g/cm3 |
| растворимость | reacts with H2O |
| форма | gas |
| Растворимость в воде | violently hydrolyzed by H2O [MER06] |
| Пределы воздействия | Ceiling 0.1 ppm (~0.4 mg/m3)(ACGIH, MSHA, NIOSH, and OSHA); IDLH 20 ppm (NIOSH). |
| Стабильность | Strong oxidizer. Incompatible with, and may ignite or react violently with, combustible materials, including most organic compounds. Decomposes in water. Incompatible with moisture. |
| Справочник по базе данных CAS | 7790-91-2(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | 921841L3N0 |
| Система регистрации веществ EPA | Chlorine trifluoride (7790-91-2) |
| Коды опасности | O | |||||||||
| Заявления о рисках | 8-35 | |||||||||
| Заявления о безопасности | 17-38 | |||||||||
| OEL | Ceiling: 0.1 ppm (0.4 mg/m3) | |||||||||
| РИДАДР | 1749 | |||||||||
| Примечание об опасности | Oxidising agent | |||||||||
| Класс опасности | 2.3 | |||||||||
| Банк данных об опасных веществах | 7790-91-2(Hazardous Substances Data) | |||||||||
| ИДЛА | 12 ppm (45 mg/m3) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)




-
сигнальный язык
опасность
-
вредная бумага
H314:При попадании на кожу и в глаза вызывает химические ожоги.
H330:Смертельно при вдыхании.
H280:Газ под давлением. Баллоны (емкости) могут взрываться при нагревании.
H270:Окислитель; может вызвать или усилить возгорание.
-
оператор предупредительных мер
P220:Не допускать соприкосновения с одеждой и другими горючими материалами.
P244:Не допускать попадания жиров и масел в редукционные клапаны.
P260:Не вдыхать газ/ пары/ пыль/ аэрозоли/ дым/ туман.
P264:После работы тщательно вымыть кожу.
P271:Использовать только на открытом воздухе или в хорошо вентилируемом помещении.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P284:Использовать средства защиты органовдыхания.
P301+P330+P331:ПРИ ПРОГЛАТЫВАНИИ: Прополоскать рот. Не вызывать рвоту!
P303+P361+P353:ПРИ ПОПАДАНИИ НА КОЖУ (или волосы): Снять/удалить немедленно всю загрязненную одежду. Промыть кожу водой.
P304+P340:ПРИ ВДЫХАНИИ: Свежий воздух, покой.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
P310:Немедленно обратиться за медицинской помощью.
P363:Перед повторным использованием выстирать загрязненную одежду.
P370+P376:При пожаре ликвидировать утечку, если это не сопряжено сриском.
P403:Хранить в хорошо вентилируемом месте.
P403+P233:Хранить в хорошо вентилируемом месте в плотно закрытой/герметичной таре.
P405:Хранить в недоступном для посторонних месте.
P410+P403:Беречь от солнечных лучей. Хранить в хорошо вентилируемом месте.
P501:Удалить содержимое/ контейнер на утвержденных станциях утилизации отходов.
ХЛОРА ТРИФТОРИД химические свойства, назначение, производство
Химические свойства
Chlorine trifluoride is a greenish yellow, almost colorless, liquid (below 12C/53F), or colorless gas with a sweet, irritating odor. Shipped as a liquefied compressed gas.Физические свойства
Colorless gas; sweetish but suffocating odor; density of the liquid 1.77 g/mL at 13°C; condenses to a greenish yellow liquid at 11.75°C; freezes to a white solid at -76.3°C; reacts violently with water.Использование
Chlorine trifluoride is used as a fluorinatingagent, as a rocket propellant, in processingof nuclear reactor fuel, and in incendiaries.It is also used as an inhibitor of pyrolysis offluorocarbon polymers.Общее описание
A colorless gas or green liquid with a pungent odor. Boils at 53°F. CHLORINE TRIFLUORIDE reacts with water to form chlorine and hydrofluoric acid with release of heat. Contact with organic materials may result in spontaneous ignition. CHLORINE TRIFLUORIDE is corrosive to metals and tissue. Prolonged exposure to low concentrations or short term exposure to high concentrations may result in adverse health effects. Under prolonged exposure to fire or intense heat the container may violently rupture and rocket.Реакции воздуха и воды
A violent reaction occurs with water or ice generating acidic HF and chlorine, [Sidgwick, 1156(1950)]. The release of CHLORINE TRIFLUORIDE to the atmosphere rapidly generates two toxic reaction products: HF and Chlorine Dioxide, [Lombardi, D.A. and M.D. Cheng 1996. "Modeling Accidental Releases of CHLORINE TRIFLUORIDE to the Atmosphere," Paper No. 96-WP66B.02, presented at the 89th Annual Meeting of the Air and Waste Management Association, Nashville, Tennessee, June 23-26].Опасность
Explodes in contact with organic materials or with water. Dangerous fire risk. A poison, very toxic, corrosive to skin. Lung damage, eye, and upper respiratory tract irritant. Questionable carcinogen.Угроза здоровью
Chlorine trifluoride is a severe irritant tothe skin, eyes, and mucous membranes.Exposure to this gas can cause lung dam age. A 30-minute exposure to 400 ppm waslethal to rats. It decomposes in the presenceof moisture to chlorine, chlorine dioxide,and hydrogen fluoride, all of which arehighly toxic. Chronic inhalation study on ani mals for a period of 6 months (6 hours/day,5 days/week) indicated that at an exposurelevel of nearly 1 ppm the early symptomswere sneezing, salivation, and expulsionof frothy fluid from the mouth and nose(ACGIH 1986). This progressed to mus cle weakness, pneumonia, and lung damage.Some animals died.In humans, exposure to this gas can pro duce severe injury to the eyes, skin, andrespiratory tract, and pulmonary edema. Theliquid is severely corrosive to the skin andeyes. Skin contact can cause painful burns.
Пожароопасность
Nonflammable gas; dangerously reactive. Chlorine trifluoride reacts explosively with water, forming hydrogen fluoride and chlo rine. It reacts violently with most elements and common substances. Paper, cloth, wood, glass, wool, charcoal, and graphite burst into flame in contact with the liquid. The vapors, even when diluted, can set fire to organic compounds. Reactions with most metals are vigorous to violent, often caus ing a fire. It catches fire when mixed with phosphorus, arsenic, antimony, silicon, sul fur, selenium, tellurium, tungsten, osmium, and rhodium (Mellor 1946, Suppl. 1956). Among the alkali- and alkaline–earth metals, reaction is violent with potassium at ordinary temperatures, and with sodium, calcium, or magnesium it reacts violently at elevated temperatures. Violent reaction occurs with oxides, sulfides, halides, and carbides of metals, causing flames. Chlorine trifluoride attacks sand, glass, and asbestos. Prolonged contact can ignite glass. Explosive reactions occur with many common gases, includ ing hydrogen, lower hydrocarbons, carbon monoxide, ammonia, hydrogen sulfide, and sulfur dioxide. Reactions with mineral acids and alkalies are violent.In case of a small fire involving chlorine trifluoride, use a dry chemical or water spray in large amounts (NFPA 1997). Allow large fires to burn. Avoid contact of chlorine trifluoride with the body or with protective clothing.
Возможный контакт
Chlorine trifluoride is used as a fluorinating agent. It may be used as an igniter and propellant in rockets. It is used in nuclear fuel processing.хранилище
Chlorine trifluoride is stored and shippedin special steel cylinders. It is stored inmoisture-free, cool, and isolated areas sepa rated from other chemicals. The cylinders arekept upright, covered, and protected againstphysical damage.Перевозки
UN1749 Chlorine trifluoride, Hazard class: 2.3; Labels: 2.3-Poisonous gas, 5.1-Oxidizer, 8-Corrosive material, Inhalation Hazard Zone B. Cylinders must be transported in a secure upright position, in a well-ventilated truck. Protect cylinder and labels from physical damage. The owner of the compressed gas cylinder is the only entity allowed by federal law (49CFR) to transport and refill them. It is a violation of transportation regulations to refill compressed gas cylinders without the express written permission of the owner.Методы очистки
Impurities include chloryl fluoride, chlorine dioxide and hydrogen fluoride. Passed it first through two U-tubes containing NaF to remove HF, then through a series of traps in which the liquid is fractionally distilled. It can be purified via the KF complex; KClF4, formed by adding excess ClF3 to solid KF in a stainless steel cylinder in a dry-box and shaking overnight. After pumping out the volatile materials, pure ClF3 is obtained by heating the bomb to 100-150o and condensing the evolved gas in a -196o trap [Schack et al. Chem Ind (London) 545 1967]. It attacks glass very vigorously. HIGHLY TOXIC.Несовместимости
A powerful oxidizer. Keep away from acids. Most combustible materials ignite spontaneously on contact with chlorine trifluoride. Explodes on contact with organic materials. The liquid can explode if mixed with halocarbons or hydrocarbons. It reacts violently with oxidizable materials, finely divided metals and metal oxides; sand, glass, asbestos, silicon-containing compounds. Emits highly toxic fumes on contact with acids. Chlorine trifluoride decomposes above 220C, forming Thermal decomposition products may include hydrogen chloride and HF. Reacts violently with water, forming chlorine gas and hydrofluoric acid. Reacts with most forms of plastics, rubber, coatings, and resins; except the highly fluorinated polymers, such as Teflon and “K el-F.”Утилизация отходов
Return refillable compressed gas cylinders to supplier.ХЛОРА ТРИФТОРИД запасные части и сырье
запасной предмет