Eptifibatide Acetate
- русский язык имя
- английское имяEptifibatide Acetate
- CAS №148031-34-9
- CBNumberCB7416565
- ФормулаC35H49N11O9S2
- мольный вес831.96
- EINECS1533716-785-6
- номер MDLMFCD05662245
- файл Mol148031-34-9.mol
химическое свойство
| Температура плавления | >220°C (dec.) |
| температура хранения | Refrigerator |
| растворимость | DMSO (Slightly), Methanol (Slightly), Water (Slightly) |
| форма | Solid |
| цвет | White to Off-White |
| ИнЧИКей | CZKPOZZJODAYPZ-ITVGJGJRNA-N |
| SMILES | N12CCC[C@H]1C(=O)N[C@@H](CSSCCC(=O)N[C@@H](CCCCNC(N)=N)C(=O)NCC(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC1C3C=CC=CC=3NC=1)C2=O)C(N)=O |&1:4,8,17,33,41,r| |
| Справочник по базе данных CAS | 148031-34-9(CAS DataBase Reference) |
| FDA UNII | NA8320J834 |
| Код УВД | B01AC16 |
| UNSPSC Code | 41116107 |
| NACRES | NA.24 |
Eptifibatide Acetate химические свойства, назначение, производство
Показания
Acute Coronary Syndrome (ACS)INTEGRILIN? is indicated to decrease the rate of a combined endpoint of death or new myocardial infarction (MI) in patients with ACS (unstable angina [UA]/non-ST-elevation myocardial infarction [NSTEMI]), including patients who are to be managed medically and those undergoing percutaneous coronary intervention (PCI).
Percutaneous Coronary Intervention (PCI)
INTEGRILIN is indicated to decrease the rate of a combined endpoint of death, new MI, or need for urgent intervention in patients undergoing PCI, including those undergoing intracoronary stenting.
прикладной
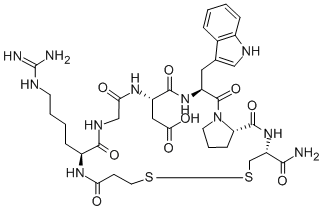
Eptifibatide is a cyclic heptapeptide containing 6 amino acids and 1 mercaptopropionyl (des-amino cysteinyl) residue. An interchain disulfide bridge is formed between the cysteine amide and the mercaptopropionyl moieties. Chemically it is N6-(aminoiminomethyl)-N2-(3-mercapto-1-oxopropyl)-Llysylglycyl-L-α-aspartyl-L-tryptophyl-L-prolyl-L-cysteinamide, cyclic (1→6)-disulfide. Eptifibatide binds to the platelet receptor glycoprotein (GP) IIb/IIIa of human platelets and inhibits platelet aggregation.The eptifibatide peptide is produced by solution-phase peptide synthesis, and is purified by preparative reverse-phase liquid chromatography and lyophilized. The structural formula is:

Лекарственные формы
Before infusion of INTEGRILIN, the following laboratory tests should be performed to identify pre-existing hemostatic abnormalities: hematocrit or hemoglobin, platelet count, serum creatinine, and PT/aPTT. In patients undergoing PCI, the activated clotting time (ACT) should also be measured.The activated partial thromboplastin time (aPTT) should be maintained between 50 and 70 seconds unless PCI is to be performed. In patients treated with heparin, bleeding can be minimized by close monitoring of the aPTT and ACT.
Eptifibatide Acetate поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| 0551-65326643 18156095617 |
China | 995 | 58 | ||
| +86-0571-85134551 | China | 15352 | 58 | ||
| +8618108235634 | China | 2345 | 58 | ||
| +8619375158599 | China | 5223 | 58 | ||
| +8615354498258 | China | 111 | 58 | ||
| +86-13131129325 | China | 5887 | 58 | ||
| 0592-5800732; +8613806035118 |
China | 988 | 58 | ||
| +86-0533-2185556 +8617865335152 |
China | 10986 | 58 | ||
| +undefined17712220823 | China | 615 | 58 | ||
| +8618092446649 | China | 1143 | 58 |