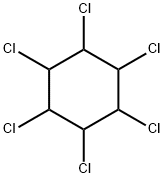
BHC
- русский язык имя
- английское имяBHC
- CAS №608-73-1
- CBNumberCB7190232
- ФормулаC6H6Cl6
- мольный вес290.83
- EINECS210-168-9
- номер MDLMFCD00003825
- файл Mol608-73-1.mol
| Температура плавления | 112.75°C |
| Температура кипения | 373.64°C (rough estimate) |
| плотность | 1.8700 |
| показатель преломления | 1.5691 (estimate) |
| растворимость | DMSO (Slightly), Methanol (Slightly) |
| форма | Powder or Flakes |
| цвет | White,crystalline powder |
| Растворимость в воде | 31.4mg/L(25 ºC) |
| FDA UNII | 5477B350EK |
| Система регистрации веществ EPA | 1,2,3,4,5,6-Hexachlorocyclohexane (608-73-1) |
| Коды опасности | T,N |
| Заявления о рисках | 21-25-40-50/53-64-48/22-20/21 |
| Заявления о безопасности | 22-36/37-45-60-61 |
| РИДАДР | 2761 |
| WGK Германия | 3 |
| RTECS | GV4375000 |
| Класс опасности | 6.1(b) |
| Группа упаковки | III |
| Банк данных об опасных веществах | 608-73-1(Hazardous Substances Data) |
| Токсичность | otr-rat-orl 875 mg/kg/7W-I CRNGDP 5,479,84 |
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)



-
сигнальный язык
опасность
-
вредная бумага
H301:Токсично при проглатывании.
H351:Предполагается, что данное вещество вызывает раковые заболевания.
H362:Может причинить вред детям, находящимся на грудном вскармливании.
H410:Чрезвычайно токсично для водных организмов с долгосрочными последствиями.
H373:Может поражать органы (Нервная система) в результате многократного или продолжительного воздействия при вдыхании.
H312:Вредно при попадании на кожу.
-
оператор предупредительных мер
P260:Не вдыхать газ/ пары/ пыль/ аэрозоли/ дым/ туман.
P263:Избегать контакта в период беременности и грудного вскармливания.
P273:Избегать попадания в окружающую среду.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P301+P310:ПРИ ПРОГЛАТЫВАНИИ: Немедленно обратиться за медицинской помощью. Прополоскать рот.
P302+P352+P312:ПРИ ПОПАДАНИИ НА КОЖУ: Промыть большим количеством воды. Обратиться за медицинской помощью при плохом самочувствии.
BHC химические свойства, назначение, производство
Химические свойства
BHC is a white-to-brownish crystalline solid with a musty, phosgene-like odor.Использование
Insecticide.Определение
An environmentally persistent constituent of a wide variety of agricultural, medical, and veterinary products.Общее описание
White to yellow powder or flakes. Musty odor. The gamma isomer is known as lindane, a systemic insecticide. Toxic by ingestion or inhalation.Профиль реактивности
Halogenated aliphatic compounds, such as HEXACHLOROCYCLOHEXANE, are moderately or very reactive. Reactivity generally decreases with increased degree of substitution of halogen for hydrogen atoms. Materials in this group may be incompatible with strong oxidizing and reducing agents. Also, they may be incompatible with many amines, nitrides, azo/diazo compounds, alkali metals, and epoxides.Опасность
Highly toxic; questionable carcinogen.Возможный контакт
The major commercial usage of BHC is based upon its insecticidal properties. α-BCH is used as an Agricultural chemical, pesticide, pharmaceutical, and veterinary drug. The 7-isomer has the highest acute toxic ity, but the other isomers are not without activity. It is gen erally advantageous to purify the 7-isomer from the less active isomers. The γ-isomer acts on the nervous system of insects, principally at the level of the nerve ganglia. As a result, lindane has been used against insects in a wide range of applications including treatment of animals, buildings, humans for ectoparasites, clothes; water for mosquitoes; living plants; seeds and soils. Some applications have been abandoned due to excessive residues, e.g., stored food stuffs. By voluntary action, the principal domestic producer of technical grade BHC requested cancellation of its BHC registrations on September 1, 1976. As of July 21, 1978, all registrants of pesticide products containing BHC voluntar ily canceled their registrations or switched their former BHC products to lindane formulations.Канцерогенность
A bioassay of lindane of the NCI was published in 1977. The compound was administered for 80 weeks to groups of Osborne–Mendel rats and B6C3F1 mice. Time-weighted dietary levels for male rats were 236 or 472 ppm; for female rats, 135 or 270 ppm; and for male and female mice, 80 or 160 ppm. Results were as follows: in rats, no tumors occurred at a statistically significant incidence in the treated groups of either sex but (2) in mice, the incidence of hepatocellular carcinoma in lowdose males was significant when compared with that in the pooled controls (controls 5/49, low dose 19/49, p = 0.001). This finding, by itself, is insufficient to establish the carcinogenicity of lindane. The incidence of hepatocellular carcinoma in high-dose male mice was not significantly different from that in matched or pooled controls. It is concluded that under the conditions of this bioassay lindane was not carcinogenic for Osborne–Mendel rats or B6C3F1 mice.As with most other chlorinated insecticides, lindane has been shown to exhibit tumor promotional activity, possibly via inhibition of intercellular communication. Lindane exposures do inhibit formation of liver tumors following exposures to aflatoxin B1, suggesting an activity other than tumor promotion for lindane. Enhancement of enzymatic deactivation of aflatoxin via induction of microsomal enzymes may play a role in this phenomenon.
The combination of lindane’s lack of significant mutagenic activity, its tumor promotional properties, and the lack of epidemiologic evidence suggest that lindane poses a minimal to nonexistent risk of cancer to humans under reasonable and prescribed use conditions.
Перевозки
UN2761 Organochlorine pesticides, solid, toxic, Hazard Class: 6.1; Labels: 6.1-Poisonous materials.Несовместимости
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explo sions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. Decomposes on contact with powdered iron, aluminum, zinc, and on contact with strong bases producing trichlorobenzene.Утилизация отходов
A process has been developed for the destructive pyrolysis of benzene hexachloride @ 400 500℃ with a catalyst mixture which contains 5 10% of either cupric chloride, ferric chloride; zinc chloride; or aluminum chloride on activated carbon.BHC поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| 86-13657291602 | CHINA | 22963 | 58 | |
| +86-057181025280; +8617767106207 |
China | 49734 | 58 | |
| +8618950047208 | China | 43416 | 58 | |
| +86-89586680 +86-13289823923 |
China | 8670 | 58 | |
| +86-400-002-6226 +86-13028896684; |
China | 57423 | 58 | |
| 18017382783 18017382783 |
China | 9611 | 58 | |
| 0755-66853366; 13670046396 |
China | 24342 | 58 | |
| 029-81120477 17792793610 |
China | 10010 | 58 | |
| 4008-099-669 | CHINA | 73043 | 58 | |
| 58896805 | CHINA | 15620 | 58 |