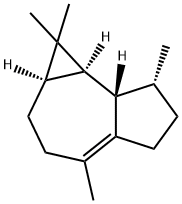
(+)-Ledene
- русский язык имя
- английское имя(+)-Ledene
- CAS №21747-46-6
- CBNumberCB7153554
- ФормулаC15H24
- мольный вес204.35
- EINECS244-565-3
- номер MDLMFCD00042613
- файл Mol21747-46-6.mol
химическое свойство
| Температура плавления | 269°C |
| Температура кипения | 268-270 °C (lit.) |
| плотность | 0.927 g/mL at 20 °C (lit.) |
| показатель преломления | n |
| температура хранения | Refrigerator |
| растворимость | Chloroform (Sparingly), Ethanol (Slightly) |
| форма | Oil |
| цвет | Colourless |
| оптическая активность | [α]20/D +68±2°, c = 10% in ethanol |
| БРН | 2554786 |
| LogP | 6.447 (est) |
| FDA UNII | 236ZZ41F70 |
| UNSPSC Code | 12352002 |
| NACRES | NA.22 |
| Заявления о безопасности | 23-24/25 |
| WGK Германия | 3 |
(+)-Ledene химические свойства, назначение, производство
Описание
(+)-Ledene, also called leden or viridiflorene, is an organic compound that belongs to the group of 5, 10-cycloaromadendrane sesquiterpenoids. These compounds are derived from the aromadendrane skeleton through a cyclization reaction between carbon atoms 5 and 10. (+)-Ledene is mainly found in saliva and is considered to have low solubility in water and a relatively neutral nature. In cellular environments, (+)-ledene is predominantly present in the cell membrane (predicted based on logP) and cytoplasm.Использование
(+)-Ledene is an essential oil that is synthesized by viridiflorene synthase. It has been studied as an antioxidant, antimicrobial, antibiofilm and to treat various cancers.Определение
ChEBI: Viridiflorene is a carbotricyclic sesquiterpene obtained from several natural sources, including Australian Tea Tree oil (Melaleuca alternifolia.) It is a sesquiterpene and a carbotricyclic compound.Общее описание
(+)-Ledene is a naturally occurring sesquiterpene that can be prepared from (+)-aromadendrene via isomerization.использованная литература
[1] DUC N. TRAN Prof.??Dr. N C. Biomimetic Synthesis of (+)-Ledene, (+)-Viridiflorol, (?)-Palustrol, (+)-Spathulenol, and Psiguadial A, C, and D via the Platform Terpene (+)-Bicyclogermacrene[J]. Chemistry - A European Journal, 2014. DOI:10.1002/chem.201403082.[2] S. L. GWALTNEY K. J S S T Sakata. Bridged to Fused Ring Interchange. Methodology for the Construction of Fused Cycloheptanes and Cyclooctanes. Total Syntheses of Ledol, Ledene, and Compressanolide[J]. The Journal of Organic Chemistry, 1996. DOI:10.1021/jo961005a.
(+)-Ledene поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| 18017610038 | CHINA | 3619 | 58 | |
| 18871490254 | CHINA | 28172 | 58 | |
| +1-781-999-5354 +1-00000000000 |
United States | 32161 | 58 | |
| +86-89586680 +86-13289823923 |
China | 8670 | 58 | |
| +8618058761490 | China | 49983 | 58 | |
| +86-18621343501; +undefined18621343501 |
China | 33338 | 58 | |
| +1-+1(833)-552-7181 | United States | 52924 | 58 | |
| +8613817748580 | China | 40066 | 58 | |
| 571-89925085 | China | 131957 | 58 | |
| 021-61259108 18621169109 |
China | 40228 | 62 |