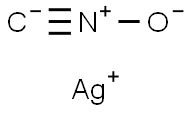
гремящее серебро
- английское имяsilver fulminate
- CAS №5610-59-3
- CBNumberCB71335904
- ФормулаCAgNO
- мольный вес149.89
- файл Mol5610-59-3.mol
химическое свойство
| FDA UNII | 5QNF7XT857 |
| Система регистрации веществ EPA | Silver fulminate (5610-59-3) |
гремящее серебро химические свойства, назначение, производство
Описание
Silver fulminate, AgCNO, a powerful, touch-sensitive explosive used in percussion caps, is prepared through the reaction of silver metal with nitric acid in the presence of ethanol.Химические свойства
White crystals of needle shape; explodeson heating; very slightly soluble in water(approximately 750 mg/L) (CRC Handbook1996); soluble in NH4OH.Использование
It is used as a detonator. A potential risk ofits formation may arise when silver metal,silver nitrate or any other silver salt is mixedwith nitric acid and ethanol..Пожароопасность
It is one of the most powerful detonating fulminates. Its detonating power and sensitivity to heat and impact are greater than for mercury fulminate. The heat of formation, ?Hz is C361.5 kJ (C86.4 kcal)/mol for the dimer (Collins 1978). Reaction with hydrogen sulfide at ambient temperature could proceed to explosive violence (Bretherick 1995). Although soluble in ammonia, however, at a pH of >13, it is likely to produce an explosive precipitate.гремящее серебро запасные части и сырье
запасной предмет