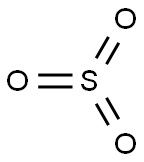
sulfur trioxide
- русский язык имя
- английское имяsulfur trioxide
- CAS №14265-45-3
- CBNumberCB71236265
- ФормулаO3S
- мольный вес80.06
- номер MDLMFCD11040984
- файл Mol14265-45-3.mol
sulfur trioxide химические свойства, назначение, производство
Описание
Endogenous sulfite is generated as a consequence of the body’’s normal processing of sulfur-containing amino acids. In addition, as discussed below, sulfite can be produced by neutrophils. Sulfites occur as a consequence of fermentation and also naturally in a number of foods and beverages. As food additives, sulfating agents were first used in 1664, and approved for use in the United States in the 1800s. Sulfite is also noted as a water treatment additive, for example, to control oxygen levels in power plant boiler water. Further, sulfur dioxide is acommonair pollutant produced by numerous processes (burning sulfurbearing coal, smelting sulfide ores, etc.), and may enter the body via inhalation. Sulfur dioxide has been reported to react with water in the ambient air and in the respiratory tract’s mucous membranes to form sulfite and bisulfite ions.Использование
Inorganic sulfites and bisulfites (such as sodium sulfite, Na2O3S) are used in photography, the bleaching of wool, and as preservatives in foods, beverages, and medications. They act as effective antioxidant compounds and are also used in the manufacture of pulp for paper and wood products. Their preservative properties include controlling microbial growth and the prevention of browning and spoilage.Under the US Federal Food, Drug, and Cosmetic Act, sulfites are permitted for use as preservatives in food. Like other ingredients, sulfites must be declared in the ingredient statement when added to a food product. In addition, sodium sulfite, ammonium sulfite, sodium bisulfite, potassium bisulfite, ammonium bisulfite, sodium metabisulfite, and potassium metabisulfite are inorganic salts that function as reducing agents in cosmetic formulations. All except sodium metabisulfite also function as hair waving/straightening agents. In addition, sodium sulfite, potassium sulfite, sodium bisulfite, and sodium metabisulfite function as antioxidants in cosmetics. All except ammonium sulfite are widely used in hair care products.
Определение
ChEBI: Sulfite is an inorganic anion, which is the conjugate base of hydrogen sulfite.Экологическая судьба
Sulfites are generally soluble compounds that interact with the environment through a variety of processes. The primary function of sulfites is that of a reducing agent, which can remove dissolved oxygen from waterways. This reduction of dissolved oxygen (normally produced via normal aeration through water movement, falls and disturbances, etc.) in turn generates a favorable environment for anaerobic bacteria, disrupting the local microbiota. Decreases in dissolved oxygen caused by the presence of sulfites, typically below 5 ppm dissolved oxygen, can negatively affect fish and other organisms present in polluted waterways. Another effect of sulfite contamination of waterways is the production of hydrogen sulfide gas, which is a by-product of sulfite-induced redox processes.Flue gas desulfurization is an industrial process that produces calcium sulfite (CaSO3) as a by-product. This relatively insoluble sulfite, when deposited in soils, can cause a general shift in local microbiot a; however, calcium sulfite readily undergoes oxidation to calcium sulfate (gypsum), which is generally accepted as a remediation material for poor quality soils. While the resulting gypsum can have beneficial effects on soils for agricultural purposes, large quantities of sulfite species over longer time spans can be expected to directly affect microbial communities in soils, even after conversion to the more benign calcium sulfate. Consequently, the presence of calcium sulfate may produce blooming as a result of oxygen- and sulfurenriched soils and adjoining waterways.
sulfur trioxide запасные части и сырье
запасной предмет