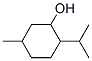
MENTHOL
- русский язык имя
- английское имяMENTHOL
- CAS №15356-70-4
- CBNumberCB7116412
- ФормулаC10H20O
- мольный вес156.27
- EINECS239-388-3
- номер MDLMFCD00001484
- файл Mol15356-70-4.mol
| Температура плавления | 34-36 °C(lit.) |
| Температура кипения | 216 °C(lit.) |
| плотность | 0.89 g/mL at 25 °C(lit.) |
| давление пара | 0.8 mm Hg ( 20 °C) |
| FEMA | 2665 | MENTHOL RACEMIC |
| показатель преломления | 1.4615 |
| Fp | 200 °F |
| температура хранения | 2-8°C |
| растворимость | Practically insoluble in water, very soluble in ethanol (96 per cent) and in light petroleum, freely soluble in fatty oils and in liquid paraffin, very slightly soluble in glycerol. |
| форма | cryst. |
| цвет | White |
| Диэлектрическая постоянная | 3.2(Ambient) |
| ИнЧИКей | NOOLISFMXDJSKH-UHFFFAOYSA-N |
| Справочник по базе данных CAS | 15356-70-4(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | YS08XHA860 |
| Коды опасности | Xn,Xi |
| Заявления о рисках | 37/38-41-48/20/22-40-38-22 |
| Заявления о безопасности | 36/37/39-36-26 |
| РИДАДР | UN 1888 6.1/PG 3 |
| WGK Германия | 2 |
| RTECS | OT0525000 |
| кода HS | 29061100 |
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
опасность
-
вредная бумага
H315:При попадании на кожу вызывает раздражение.
H319:При попадании в глаза вызывает выраженное раздражение.
H302:Вредно при проглатывании.
H372:Поражает органы в результате многократного или продолжительного воздействия.
H351:Предполагается, что данное вещество вызывает раковые заболевания.
H336:Может вызывать сонливость или головокружение.
H361d:Предполагается, что данное вещество может отрицательно повлиять на неродившегося ребенка.
H331:Токсично при вдыхании.
-
оператор предупредительных мер
P202:Перед использованием ознакомиться с инструкциями по технике безопасности.
P301+P312:ПРИ ПРОГЛАТЫВАНИИ: Обратиться за медицинской помощью при плохом самочувствии.
P302+P352:ПРИ ПОПАДАНИИ НА КОЖУ: Промыть большим количеством воды.
P304+P340+P311:ПРИ ВДЫХАНИИ: Свежий воздух, покой. Обратиться за медицинской помощью.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
P308+P313:ПРИ подозрении на возможность воздействия обратиться за медицинской помощью.
MENTHOL химические свойства, назначение, производство
Химические свойства
Racemic menthol is a mixture of equal parts of the (1R,2S,5R)- and (1S,2R,5S)-isomers of menthol. It is a free-flowing or agglomerated crystalline powder, or colorless, prismatic, or acicular shiny crystals, or hexagonal or fused masses with a strong characteristic odor and taste. The crystalline form may change with time owing to sublimation within a closed vessel. The USP 32 specifies that menthol may be either naturally occurring l-menthol or syntheti-cally prepared racemic or dl-menthol. However, the JP XV and PhEur 6.0, along with other pharmacopeias, include two separate monographs for racemic and l-menthol.Физические свойства
Physical Properties. The eight optically active menthols differ in their sensory properties. (?)-Menthol has a characteristic peppermint odor and also exerts a cooling effect. The other isomers do not possess this cooling effect and are, therefore, not considered to be “refreshing.” Racemic menthol occupies an intermediate position; the cooling effect of the (?)-menthol present is distinctly perceptible.The enantiomeric menthols have identical physical properties (apart from their specific rotations), but the racemates differ from the optically active forms in, for example, theirmelting points.Although the differences between the boiling points are small, the (racemic) stereoisomers can be separated by fractional distillation. Boiling points (in °C at 101.3 kPa) are as follows:
rac-neomenthol, 211.7
rac-neoisomenthol 214.6
rac-menthol, 216.5
rac-isomenthol, 218.6
Other physical constants of commercially available levorotatory and racemic menthols are as follows: (?)-menthol, mp 43 °C, [α]20 D ?50° (ethanol, 10%); racemic menthol, mp 38 °C.
Chemical Properties. Hydrogenation of menthols yields p-menthane; oxidation with chromic acid or catalytic dehydrogenation yields menthones. Dehydration under mild conditions yields 3-p-menthene as the main product. Reaction with carboxylic acids or their derivatives yields menthyl esters, which are used mainly as aroma substances and in pharmaceutical preparations and formulations. The esterification of menthols with benzoic acid is used on an industrial scale in the resolution of racemic menthol.
Вхождение
Has apparently not been reported to occur in natureПодготовка
By hydrogenation of thymol followed by separation from its other isomers.Показания
Menthol, a cyclic alcohol (derived from peppermint, other mint oils, or prepared synthetically), relieves itching by generating a cool sensation. It is usually used in 0.25% to 2% concentrations but is present in concentrations as high as 16% in some OTC products.Методы производства
Menthol occurs widely in nature as l-menthol and is the principal component of peppermint and cornmint oils obtained from the Mentha piperita and Mentha arvensis species. Commercially, lmenthol is mainly produced by extraction from these volatile oils. It may also be prepared by partial or total synthetic methods.Racemic menthol is prepared synthetically via a number of routes, e.g. by hydrogenation of thymol.
Фармацевтические приложения
Menthol is widely used in pharmaceuticals, confectionery, and toiletry products as a flavoring agent or odor enhancer. In addition to its characteristic peppermint flavor, l-menthol, which occurs naturally, also exerts a cooling or refreshing sensation that is exploited in many topical preparations. Unlike mannitol, which exerts a similar effect due to a negative heat of solution, l-menthol interacts directly with the body’s coldness receptors. d-Menthol has no cooling effect, while racemic menthol exerts an effect approximately half that of l-menthol.When used to flavor tablets, menthol is generally dissolved in ethanol (95%) and sprayed onto tablet granules and not used as a solid excipient.
Menthol has been investigated as a skin-penetration enhancer and is also used in perfumery, tobacco products, chewing gum and as a therapeutic agent. When applied to the skin, menthol dilates the blood vessels, causing a sensation of coldness followed by an analgesic effect. It relieves itching and is used in creams, lotions, and ointments. When administered orally in small doses menthol has a carminative action.
Профиль безопасности
Moderately toxic by ingestion, intraperitoneal, and subcutaneous routes. An eye and skin irritant. K%en heated to decomposition it emits acrid smoke and irritating fumes. See also MENTHOL and 1-MENTHOL.Безопасность
Almost all toxicological data for menthol relate to its use as a therapeutic agent rather than as an excipient. Inhalation or ingestion of large quantities can result in serious adverse reactions such as ataxia and CNS depression,hypersensitivity reactions, severe abdominal pain, nausea, vomiting, vertigo, drowsiness, and coma.Although menthol is essentially nonirritant there have been some reports of hypersensitivity following topical application. In a Polish study approximately 1% of individuals were determined as being sensitive to menthol.There have been reports of apnea and instant collapse in infants after the local application of menthol to their nostrils.The WHO has set an acceptable daily intake of menthol at up to 0.4 mg/kg body-weight.
LD50 (rat, IM): 10.0 g/kg
LD50 (rat, oral): 3.18 g/kg
Метаболизм
Rabbits are said to eliminate 59% of dl-menthol as glucuronide (Williams, 1938).хранилище
A formulation containing menthol 1% w/w in aqueous cream has been reported to be stable for up to 18 months when stored at room temperature.Menthol should be stored in a well-closed container at a temperature not exceeding 25°C, since it sublimes readily.
Несовместимости
Incompatible with: butylchloral hydrate; camphor; chloral hydrate; chromium trioxide; b-naphthol; phenol; potassium permanganate; pyrogallol; resorcinol; and thymol.Регуляторный статус
Included in the FDA Inactive Ingredients Database (dental preparations, inhalations, oral aerosols, capsules, solutions, suspensions, syrups, and tablets; also topical preparations). Included in nonparenteral medicines licensed in the UK. Accepted for use in foods and confectionery as a flavoring agent of natural origin. Included in the Canadian List of Acceptable Non-medicinal Ingredients.MENTHOL запасные части и сырье
сырьё
MENTHOL поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +8615531157085 | China | 8804 | 58 | |
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | |
| +16837774622 | China | 295 | 58 | |
| +86-13591747876 +86-13591747876 |
China | 299 | 58 | |
| +86-16264648883 +86-16264648883 |
China | 3712 | 58 | |
| +86-(0)57185586718 +86-13336195806 |
China | 29792 | 60 | |
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| 008657128800458; +8615858145714 |
China | 7738 | 55 | |
| +86-0551-65418679 +8618949832763 |
China | 2986 | 55 | |
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 |