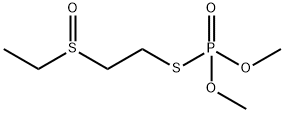
Oxydemeton-methyl
- русский язык имя
- английское имяOxydemeton-methyl
- CAS №301-12-2
- CBNumberCB7115945
- ФормулаC6H15O4PS2
- мольный вес246.28
- EINECS206-110-7
- номер MDLMFCD00055488
- файл Mol301-12-2.mol
химическое свойство
| Температура плавления | <-20℃ |
| Температура кипения | 106 °C(Press: 0.01 Torr) |
| плотность | 1.289 g/cm3 (20 ºC) |
| давление пара | 3.8×10-3 Pa (20°C) |
| показатель преломления | 1.5216 (589.3 nm 20℃) |
| температура хранения | 0-6°C |
| растворимость | Chloroform (Sparingly), Ethyl Acetate (Slightly), Methanol (Slightly) |
| Растворимость в воде | Miscible |
| форма | Oil |
| цвет | Colourless to Light Yellow |
| Рейтинг продуктов питания EWG | 5-6 |
| FDA UNII | E2031449YZ |
| Предложение 65 Список | Oxydemeton Methyl |
| Система регистрации веществ EPA | Oxydemeton-methyl (301-12-2) |
| UNSPSC Code | 41116107 |
| NACRES | NA.24 |
| Коды опасности | T,N |
| Заявления о рисках | 24/25-50 |
| Заявления о безопасности | 23-36/37-45-61 |
| РИДАДР | 3018 |
| RTECS | TG1420000 |
| Класс опасности | 6.1(a) |
| Группа упаковки | II |
| Банк данных об опасных веществах | 301-12-2(Hazardous Substances Data) |
| Токсичность | LC50 (96-hour) for rainbow trout 4.0 mg/L (Walker, 1964); LC50 (24-hour) for rainbow trout and bluegill sunfish 10 mg/L (Hartley and Kidd, 1987); acute oral LD50 for rats 65–75 mg/kg (Hartley and Kidd, 1987), 30 mg/kg (RTECS, 1985). |
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
опасность
-
вредная бумага
H400:Чрезвычайно токсично для водных организмов.
H301+H311:Токсично при проглатывании или при контакте с кожей.
-
оператор предупредительных мер
P273:Избегать попадания в окружающую среду.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P301+P310:ПРИ ПРОГЛАТЫВАНИИ: Немедленно обратиться за медицинской помощью. Прополоскать рот.
P312:Обратиться за медицинской помощью при плохом самочувствии.
Oxydemeton-methyl химические свойства, назначение, производство
Описание
Oxydemeton-methyl, i.e., demeton-S-methyl sulfoxide, is a colorless oil, bp 106 ?C/0.01 mm Hg, vp 3.8 mPa (20 ?C). It is miscible with water and soluble in most organic solvents, except petroleum ether. Log Kow = ?0.74(21 ?C). Oxydemeton-methyl is relatively stable in acidic media but hydrolyzed in alkalinemedia;DT50 values (22 ?C) at pH 4, 7, and 9 are 107, 46, and 2 d, respectively.Химические свойства
Oxydemeton methyl is a colorless to amber-colored liquid. Oxydemeton methyl is relatively slowly hydrolyzed in acidic media, but rapidly hydrolyzed in alkaline media.Oxydemeton methyl is miscible with water; readily soluble (10–100 g/ 100 mL) in dichloromethane, 2-propanol, and toluene; and practically insoluble (<1 g/100 mL) in n-hexane.
Использование
Oxydemeton-methyl is used to control sucking insects on fruit, vines, vegetables, cereals and ornamentals.Общее описание
Clear amber liquid.Реакции воздуха и воды
Water soluble. DEMETON-S-METHYL SULFOXIDE may be rapidly hydrolyzed by alkali .Профиль реактивности
Organophosphates such as DEMETON-S-METHYL SULFOXIDE are susceptible to formation of highly toxic and flammable phosphine gas in the presence of strong reducing agents such as hydrides. Partial oxidation by oxidizing agents may result in the release of toxic phosphorus oxides.Пожароопасность
DEMETON-S-METHYL SULFOXIDE is flammable.Профиль безопасности
Poison by ingestion, skin contact, intravenous, and intraperitoneal routes. Human mutation data reported. When heated to decomposition it emits very toxic fumes of POx and SOx. See also other demeton entries.Экологическая судьба
Soil. The sulfoxide group is oxidized to the sulfone and oxidative and hydrolytic cleavage of the side chain gives dimethylphosphoric and phosphoric acids (Hartley and Kidd, 1987). Oxamyl was degraded by the microorganism Pseudomonas putida in a laboratory study using cultured bacteria (Zeigler, 1980).Plant. In asparagus, oxydemeton-methyl was converted to the corresponding sulfone (Szeto and Brown, 1982).
Chemical/Physical. Oxydemeton-methyl can be converted to the corresponding sulfone by hydrogen peroxide (Cremlyn, 1991). Emits toxic fumes of phosphorus and sulfur oxides when heated to decomposition (Sax and Lewis, 1987).
Метаболический путь
Oxydemeton-methyl is the primary thiooxidation metabolite of demeton- S-methyl. It is further oxidised rapidly to demeton-Smethylsulfon in all media. The major route of further metabolism of both sulfoxide and sulfone is via hydrolysis to the sulfoxide and sulfone thiols. Stage I1 metabolism of these thiol hydrolysis products proceeds via oxidation and conjugation and is different in soil, plants and animals. In plants the thiols are conjugated, whereas in animals they are mainly methylated and oxidised to sulfoxides, sulfones and sulfonic acids. Demethylation is an important route in mammals; however, the compound is sufficiently polar for much to be excreted in the urine unchanged. Demethylation has also been reported to occur in plants.Метаболизм
Almost 99% of orally administered oxydemeton-methyl to animals is excreted within 48 h in the urine. It is oxidized to the sulfone, followed by hydrolytic cleavage of the P?S bond. The thiol metabolites are conjugated or methylated. O-Demethylation is also an important degradation route both inmammals and plants. Oxydemeton-methyl is rapidly degraded in soils.Oxydemeton-methyl запасные части и сырье
сырьё