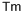
Тулий
- английское имяTHULIUM
- CAS №7440-30-4
- CBNumberCB6351960
- ФормулаTm
- мольный вес168.93
- EINECS231-140-2
- номер MDLMFCD00011281
- файл Mol7440-30-4.mol
| Температура плавления | 1545 °C (lit.) |
| Температура кипения | 1950 °C (lit.) |
| плотность | 9.332 g/mL at 25 °C (lit.) |
| температура хранения | 15-25°C |
| растворимость | soluble in dilute acid solutions |
| форма | powder |
| Удельный вес | 9.332 |
| цвет | Silver-gray |
| РН | 0.5 (20°C in H2O) |
| удельное сопротивление | 90 μΩ-cm, 20°C |
| Растворимость в воде | slowly reacts with H2O; soluble in dilute acids [HAW93] |
| Чувствительный | Air & Moisture Sensitive |
| Мерк | 13,9471 |
| Пределы воздействия | ACGIH: TWA 2 ppm; STEL 4 ppm OSHA: TWA 2 ppm(5 mg/m3) NIOSH: IDLH 25 ppm; TWA 2 ppm(5 mg/m3); STEL 4 ppm(10 mg/m3) |
| Справочник по базе данных CAS | 7440-30-4(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | 8RKC5ATI4P |
| Система регистрации веществ EPA | Thulium (7440-30-4) |
| UNSPSC Code | 41116107 |
| NACRES | NA.24 |
| Коды опасности | F,Xi,C | |||||||||
| Заявления о рисках | 15-17-36/37-34-23/24/25 | |||||||||
| Заявления о безопасности | 26-36-43-45-36/37/39-27 | |||||||||
| РИДАДР | UN 3089 4.1/PG 2 | |||||||||
| WGK Германия | 3 | |||||||||
| F | 10 | |||||||||
| TSCA | Yes | |||||||||
| Класс опасности | 4.1 | |||||||||
| Группа упаковки | II | |||||||||
| кода HS | 28053090 | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
опасность
-
вредная бумага
H319:При попадании в глаза вызывает выраженное раздражение.
H335:Может вызывать раздражение верхних дыхательных путей.
H228:Воспламеняющееся твердое вещество.
H260:При контакте с водой выделяет воспламеняющиеся газы, способные к спонтанному возгоранию.
-
оператор предупредительных мер
P210:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P223:Не допускать контакта с водой.
P231+P232:Обращаться с продуктом и хранить его в атмосфере инертного газа . Беречь от влаги.
P240:Заземлить и электрически соединить контейнер и приемное оборудование.
P261:Избегать вдыхания пыли/ дыма/ газа/ тумана/ паров/ аэрозолей.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
Тулий химические свойства, назначение, производство
Химические свойства
grey chipsФизические свойства
Thulium is a naturally occurring rare metal that exists is very small amounts mixed withother rare-earths. It is a bright silvery metal that is malleable and ductile and can be cuteasily with a knife. Its melting point is so high that it is difficult to force it into a meltedstate. Its vapor pressure is also high, and thus, much of the molten thulium evaporates intothe atmosphere. Its melting point is 1,545°C, its boiling point is 2,950°C, and its density is9.32g/cm3.Изотопы
There are a total of 46 isotopes of thulium. One of these, Tm-169 is the onlystable isotope of thulium and accounts for the total atomic mass of the element. All theother isotopes are artificially produced and radioactive and have half-lives ranging from afew microseconds to two years.Происхождение имени
Named for Thule, the Greek word for Scandinavia, the most northerly habitable land in ancient mythology.Вхождение
Thulium is the 61st most abundant element in the Earth’s crust and is found along withother rare-earths in monazite sand, which is about 50% rare-earths by weight. Only about0.007% of this is thulium. It is also found in bastnasite ore. It ranks 16th out of the 17 rareearthsin abundance. Thulium is usually found as an oxide along with other rare-earths. Likemost rare-earths, thulium can be separated from its ore by the ion-exchange process, whereits positive ion reacts with elements with negative ions like fluorine, chlorine, or oxygen toform binary compounds (e.g., Tm2O2). It can also be recovered as a by-product of the nuclearfission reaction in nuclear reactors.Характеристики
Thulium is near the end of the lanthanide series, where the metals tend to be heavier thanthe ones located near the beginning of the series. It is so scarce that it requires the processing ofabout 500 tons of earth to extract four kilograms of thulium. The only element that is scarceris promethium, which is not found naturally on Earth.История
Discovered in 1879 by Cleve. Thulium occurs in small quantities along with other rare earths in a number of minerals. It is obtained commercially from monazite, which contains about 0.007% of the element. Thulium is the least abundant of the rare-earth elements, but with new sources recently discovered, it is now considered to be about as rare as silver, gold, or cadmium. Ion-exchange and solvent extraction techniques have recently permitted much easier separation of the rare earths, with much lower costs. Only a few years ago, thulium metal was not obtainable at any cost; in 1996 the oxide cost $20/g. Thulium metal powder now costs $70/g (99.9%). Thulium can be isolated by reduction of the oxide with lanthanum metal or by calcium reduction of the anhydrous fluoride. The pure metal has a bright, silvery luster. It is reasonably stable in air, but the metal should be protected from moisture in a closed container. The element is silver-gray, soft, malleable, and ductile, and can be cut with a knife. Forty-one isotopes and isomers are known, with atomic masses ranging from 146 to 176. Natural thulium, which is 100% 169Tm, is stable. Because of the relatively high price of the metal, thulium has not yet found many practical applications. 169Tm bombarded in a nuclear reactor can be used as a radiation source in portable X-ray equipment. 171Tm is potentially useful as an energy source. Natural thulium also has possible use in ferrites (ceramic magnetic materials) used in microwave equipment. As with other lanthanides, thulium has a low-to-moderate acute toxicity rating. It should be handled with care.Использование
Thulium(III) carbonate hydrate has specialized uses in ceramics, glass, phosphors, lasers, and also is the important dopant for fibre amplifiers. Thulium(III) carbonate hydrate has use in ferrites, ceramic magnetic materials that are used in microwave equipment.Определение
thulium: Symbol Tm. A soft greymetallic element belonging to thelanthanoids; a.n. 69; r.a.m. 168.934;r.d. 9.321 (20°C); m.p. 1545°C; b.p.1947°C. It occurs in apatite and xenotime.There is one natural isotope,thulium–169, and seventeen artificialisotopes have been produced. Thereare no uses for the element, whichwas discovered by Per Cleve (1840–1905) in 1879.прикладной
Thulium products are mainly used in making crystal and lasers.An important application of the thulium in the Medicine area, and relatively independent of its high cost, is the production of portable X-ray sources. These sources are available for about one year, as tools in medical and dental diagnosis, as well as to detect defects in mechanical and electronic inaccessible components. This type of sources does not need excessive protection. Usually a small cap of lead is enough. Thulium can also be used in magnetic and ceramic materials (ferrite), similar to the Yttrium-iron alloys, nowadays used in the microwave technologies.
Thulium Metal, is mainly used in making superalloys, and has some application in ferrites (ceramic magnetic materials) used in microwave equipment and also as a radiation source of portable X-ray. Thulium potentially has use in ferrites, ceramic magnetic materials that are used in microwave equipment. it is used in arc lighting for its unusual spectrum.

Опасность
The dust and powder of thulium are explosive and toxic if inhaled or ingested. As with allradioactive elements, thulium can cause radiation poisoning.Тулий запасные части и сырье
запасной предмет
Тулий поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| 18871490254 | CHINA | 28172 | 58 | |
| +86-0551-65418671 +8618949823763 |
China | 34563 | 58 | |
| +86-029-89586680 +86-18192503167 |
China | 7724 | 58 | |
| 02120970332; +8613524231522 |
China | 2904 | 58 | |
| United States | 24072 | 58 | ||
| +86-0311-87836622 +86-17333973358 |
China | 8051 | 58 | |
| +86-18621343501; +undefined18621343501 |
China | 33338 | 58 | |
| +1-+1(833)-552-7181 | United States | 57505 | 58 | |
| +8613817748580 | China | 40066 | 58 | |
| +8618950047208 | China | 43416 | 58 |