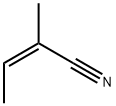
(Z) -2-метил-2-бутеннитрил
- английское имя(Z)-2-methyl-2-butenenitrile
- CAS №20068-02-4
- CBNumberCB5937640
- ФормулаC5H7N
- мольный вес81.12
- EINECS243-496-6
- номер MDLMFCD18967868
- файл Mol20068-02-4.mol
химическое свойство
| Температура кипения | 121-122 °C(Press: 772 Torr) |
| плотность | 0.81968 g/cm3 |
| FDA UNII | RKI9CB99KR |
| Система регистрации веществ EPA | cis-2-Methyl-2-butenenitrile (20068-02-4) |
| Банк данных об опасных веществах | 20068-02-4(Hazardous Substances Data) |
(Z) -2-метил-2-бутеннитрил химические свойства, назначение, производство
Реакции воздуха и воды
Highly flammable.Профиль реактивности
Nitriles, such as (Z)-2-methyl-2-butenenitrile , may polymerize in the presence of metals and some metal compounds. They are incompatible with acids; mixing nitriles with strong oxidizing acids can lead to extremely violent reactions. Nitriles are generally incompatible with other oxidizing agents such as peroxides and epoxides. The combination of bases and nitriles can produce hydrogen cyanide. Nitriles are hydrolyzed in both aqueous acid and base to give carboxylic acids (or salts of carboxylic acids). These reactions generate heat. Peroxides convert nitriles to amides. Nitriles can react vigorously with reducing agents. Acetonitrile and propionitrile are soluble in water, but nitriles higher than propionitrile have low aqueous solubility. They are also insoluble in aqueous acids.Пожароопасность
Flash point data for (Z)-2-methyl-2-butenenitrile are not available, however (Z)-2-methyl-2-butenenitrile is probably combustible.(Z) -2-метил-2-бутеннитрил запасные части и сырье
запасной предмет