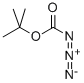
трет-бутилазидоформиат
- английское имяtert-butyl azidoformate
- CAS №1070-19-5
- CBNumberCB5892220
- ФормулаC5H9N3O2
- мольный вес143.14386
- EINECS213-972-8
- номер MDLMFCD01742129
- файл Mol1070-19-5.mol
химическое свойство
| Температура кипения | 261.22°C (rough estimate) |
| плотность | 1.3172 (rough estimate) |
| показатель преломления | 1.6190 (estimate) |
| Система регистрации веществ EPA | Carbonazidic acid, 1,1-dimethylethyl ester (1070-19-5) |
трет-бутилазидоформиат химические свойства, назначение, производство
Использование
t-Butyl azidoformate is a convenient reagent for the acylation of amines, hydrazines, and similar compounds.Подготовка
tert-Butyl chloroformate was prepared in solution as follows. Dry phosgene was introduced into a solution of 18 g (0.24 mol) of tert-butyl alcohol in 500 ml of anhydrous ether until about 52 g (0.5 mol) had been absorbed and the mixture was cooled in a Dry Ice-acetone bath. Then a solution of 20 g (0.28 mol) of pyridine in 200 ml of anhydrous ether was added dropwise with vigorous stirring. The reaction mixture was stored overnight in a Dry Ice box. The precipitated pyridine hydrochloride was filtered and the volume of the filtrate was reduced to -70 ml at reduced pressure with cooling in an icewater bath.This cold solution of tert-butyl chloroformate was added over 30 min to a vigorously stirred solution of 31.6 g (0.2 mol) of tetramethylguanidinium azide in 200 ml of chloroform; the temperature was kept at 0°C throughout the addition. The bath was removed and the reaction mixture stirred for an additional hour and then poured into 500 nil of ice water containing -2 ml of acetic acid. Extraction with two 60-ml portions of ether followed by careful evaporation of the dried (magnesium sulfate) organic phase gave tert-butyl azidoformate as a pale amber liquid in quantitative yield.The Direct Preparation of tert-Butyl Azidoformate
Ссылки на синтез
Journal of the American Chemical Society, 81, p. 955, 1959 DOI: 10.1021/ja01513a049Tetrahedron Letters, 25, p. 3701, 1984 DOI: 10.1016/0040-4039(84)80109-4
Профиль безопасности
An unstable shockand heat-sensitive explosive. It may explode above 100°C and iptes at 143°C. When heated to decomposition it emits toxic fumes of NOx. See also AZIDES.