![[[дифтор(триметилсилил)метил]тио]-бензол структурированное изображение](/CAS/GIF/536975-49-2.gif)
[[дифтор(триметилсилил)метил]тио]-бензол
- английское имя[[difluoro(triMethylsilyl)Methyl]thio]-Benzene
- CAS №536975-49-2
- CBNumberCB52644988
- ФормулаC10H14F2SSi
- мольный вес232.37
- номер MDLMFCD19442557
- файл Mol536975-49-2.mol
химическое свойство
| растворимость | soluble in most organic solvents, for example hexane, tetrahydrofuran (THF), CH2Cl2, dimethylformamide (DMF), and so on. |
[[дифтор(триметилсилил)метил]тио]-бензол химические свойства, назначение, производство
Описание
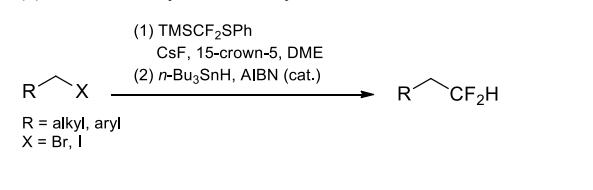
An effective reagent to introduce difluoromethyl groups into carbonyls, imines, enamines, and alkyl halides. Not only various simple aldehydes and ketones, but also functionalized carbonyls such as α- and γ-ketoesters and cyclic imides can be difluoro(phenylthio)methylated in high yields under the activation of a catalytic amount of Lewis bases. The substitution reaction proceeds well with primary alkyl bromides and iodides as the limiting reactant when cesium fluorode/15-crown-5 is used as the fluoride source/additive. Under radical conditions, the difluoro(phenylthio)methyl compounds containing vinyl functional groups can form 5- or 6-membered rings via intramolecular cyclization.Физические свойства
colorless liquid; bp 86–87?C/4 mmHg.Использование
Organofluorine compounds have received remarkable interest in recent years due to their wide-ranging biological effects. The development of general synthetic routes to such compounds and the use of new fluorinated compounds as building blocks are of great importance. Of particular interest is the selective incorporation of the gem-difluoromethylene group ‘CF2’ into organic molecules. The report on the synthesis of PhSCF2SiMe3 was published in 2003 by Prakash et al., and the reagent has become one of the most versatile and efficient nucleophilic (phenylthio)difluoromethylating reagents.When excess potassium t-butoxide was used as a promoter, PhSCF2SiMe3 reacted with diphenyl disulfide to give the corresponding dithioacetal in 85% yield (eq 8).
Подготовка
[difluoro(phenylthio)methyl]trimethylsilane (PhSCF2SiMe3) was prepared for the first time by Prakash et al.,1 using the Barbier coupling reaction of bromodifluoromethylphenyl sulfide,2 prepared from dibromodifluoromethane and sodium benzenethiolate,3 magnesium metal, and chlorotrimethylsilane (TMSCl) in DMF (eq 1).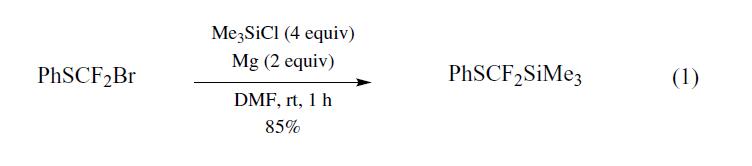
Реакции
(1) Difluoromethylation of aldehydes and ketones.
(2) Difluoromethylation of imines and enamines.

(3) (Phenylthio)difluoromethylation of imines for further cyclizations.
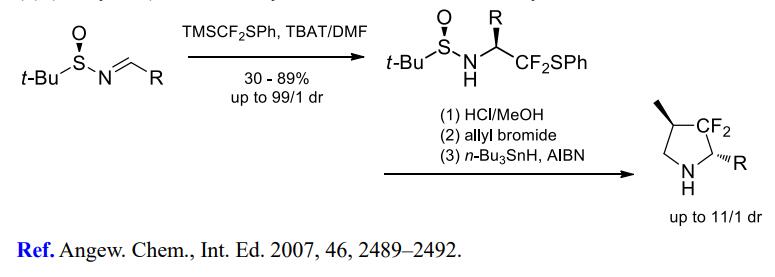
(4) Difluoromethylation of alkyl halides.
[[дифтор(триметилсилил)метил]тио]-бензол запасные части и сырье
запасной предмет
[[дифтор(триметилсилил)метил]тио]-бензол поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86-0533-2185556 +8617865335152 |
China | 10986 | 58 | |
| 010-82848833 400-666-7788 |
China | 94657 | 76 | |
| 021-50460086-9 15921403865 |
China | 6791 | 65 | |
| 010-61136123 | China | 4049 | 58 | |
| 10106090 | China | 9981 | 58 | |
| +86-400-002-6226 +86-13028896684; |
China | 57423 | 58 | |
| 025-66110311 13155353615 |
China | 15527 | 58 | |
| 18616377689 | China | 20010 | 58 | |
| 15895968936 | China | 9977 | 58 | |
| 021-021-61109150 | China | 31121 | 58 |