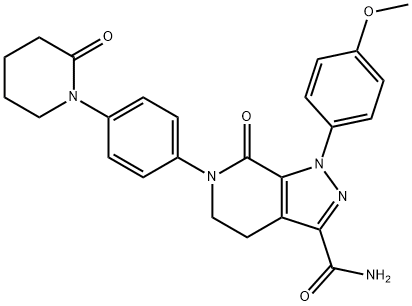
Апиксабан
- английское имяApixaban
- CAS №503612-47-3
- CBNumberCB51508586
- ФормулаC25H25N5O4
- мольный вес459.5
- EINECS639-684-6
- номер MDLMFCD11977295
- файл Mol503612-47-3.mol
| Температура плавления | 235-238°C |
| Температура кипения | 770.5±60.0 °C(Predicted) |
| плотность | 1.42 |
| температура хранения | Refrigerator |
| растворимость | DMSO (Slightly, Heated), Methanol (Slightly) |
| пка | 15.01±0.20(Predicted) |
| форма | Solid |
| цвет | White to Off-White |
| ИнЧИКей | QNZCBYKSOIHPEH-UHFFFAOYSA-N |
| SMILES | C1(=O)N(C2=CC=C(N3CCCCC3=O)C=C2)CCC2C(C(N)=O)=NN(C3=CC=C(OC)C=C3)C1=2 |
| FDA UNII | 3Z9Y7UWC1J |
| Словарь наркотиков NCI | apixaban |
| Код УВД | B01AF02 |
| UNSPSC Code | 41116107 |
| NACRES | NA.77 |
| Банк данных об опасных веществах | 503612-47-3(Hazardous Substances Data) |
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H361:Предполагается, что данное вещество может отрицательно повлиять на способность к деторождению или на неродившегося ребенка.
-
оператор предупредительных мер
P201:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P202:Перед использованием ознакомиться с инструкциями по технике безопасности.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P308+P313:ПРИ подозрении на возможность воздействия обратиться за медицинской помощью.
P405:Хранить в недоступном для посторонних месте.
P501:Удалить содержимое/ контейнер на утвержденных станциях утилизации отходов.
Апиксабан химические свойства, назначение, производство
Описание
Eliquis (apixaban), a direct inhibitor of factor Xa (FXa), was approved by the European Commission on May 18, 2011 for prevention of venous thromboembolic events (VTE) in adult patients who have undergone elective hip or knee replacement surgery. The discovery of apixaban was the culmination of a succession of novel and innovative medicinal chemistry discoveries starting with the identification of nonpeptide leads, rational drug design using computer-aided and X-ray crystallographic information, and the building of drug-like properties through the systematic replacement of basic groups with neutral moieties. Apixaban arose from modifications to razaxaban by constraining a pyrazole amide to form a bicyclic pyrazolo-pyridinone scaffold. Optimization of the P1 group resulted in the identification of the nonbasic methoxy phenyl group, while a P4 piperidinone improved the balance of potency and pharmacokinetics with low Vdss. The synthesis of apixaban begins with the generation of a hydrazone of 4-methoxyaniline which is then used in a 3+2 cycloaddition with a dihydropiperidinone to form a bicyclic pyrazolo-pyridinone scaffold. The distal piperidinone group is installed using an Ullmann coupling reaction followed by aminolysis of an ethyl ester on the pyrazole ring to complete the synthesis of apixaban.Использование
Apixaban is a highly selective, reversible inhibitor of Factor Xa with Ki of 0.08 nM and 0.17 nM in human and rabbit, respectively.Определение
ChEBI: A pyrazolopyridine that is 7-oxo-4,5,6,7-tetrahydro-1H-pyrazolo[3,4-c]pyridine-3-carboxamide substituted at position 1 by a 4-methoxyphenyl group and at position 6 by a 4-(2-oxopiperidin-1-yl)phenyl group. It is used for the prevention and treatment of thromboembolic diseases.Фармакокине?тика
The maximum plasma concentration (Cmax) of apixaban occurs 3–4 h after oral administration. The absorption of apixaban appears to occur primarily in the small intestine and decreases progressively throughout the gastrointestinal tract. Compared with oral administration, the bioavailability of 2.5 mg of apixaban solution was approximately 60% and 84% lower when released in the distal small bowel and ascending colon, respectively. For oral doses up to 10 mg, the absolute bioavailability of apixaban is~50%, resulting from the incomplete absorption and first-pass metabolism in the gut and liver[1].Клиническое использование
Apixaban is an oral anticoagulant with highly selective inhibition of factor Xa. It was approved by the European Medicines Agency (EMA) for the treatment of venous thromboembolic events and first marketed in Germany under the brand name Eliquis in June 2011. Apixaban was co-developed by Bristol-Myers Squibb and Pfizer and represents the first approved drug for this indication since warfarin over 50 years ago.Побочные эффекты
Possible side effects of Apixaban are: bleeding gums, nosebleeds, heavy vaginal bleeding , red, pink, or brown urine; red or black, tarry stools; coughing or spitting up blood or a substance that looks like coffee grounds; swelling or joint pain, headache, rash, chest pain or tightness in the chest, swelling of the face or tongue, trouble breathing, wheezing. Feeling dizzy or fainting.Apixaban prevents your blood from clotting properly, so if you get a cut or injury, it may take longer than usual for the bleeding to stop. This medication may also cause you to bruise or bleed more easily.использованная литература
https://en.wikipedia.org/wiki/Apixabanhttps://www.drugbank.ca/drugs/DB06605
[1] Wonkyung Byon. “Apixaban: A Clinical Pharmacokinetic and Pharmacodynamic Review.” Clinical Pharmacokinetics 58 10 (2019): 1265–1279.
Апиксабан поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| 0531-88110457; +8615562555968 |
China | 260 | 58 | ||
| +86-0571-85134551 | China | 15352 | 58 | ||
| 18758118018 | China | 255 | 58 | ||
| +86-852-30606658 | China | 43340 | 58 | ||
| +86-021-58950125 +86-021-58950125; |
China | 780 | 58 | ||
| +86-86-1913198-3935 +8617331935328 |
China | 970 | 58 | ||
| +86-0086-57187702781 +8613675893055 |
China | 295 | 58 | ||
| +8615531157085 | China | 8804 | 58 | ||
| +8617531153977 | China | 5855 | 58 | ||
| 0592-5800732; +8613806035118 |
China | 988 | 58 |