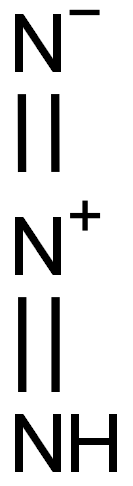
Hydrazoic acid
- русский язык имя
- английское имяHydrazoic acid
- CAS №7782-79-8
- CBNumberCB4851794
- ФормулаHN3
- мольный вес43.02804
- EINECS231-965-8
- файл Mol7782-79-8.mol
химическое свойство
| Температура плавления | -80° |
| Температура кипения | bp 37° |
| плотность | 1.092 |
| растворимость | soluble in H2O |
| пка | 4.72(at 25℃) |
| форма | colorless liquid |
| цвет | colorless liquid; explodes, explosive |
| РН | pKa1= 4.43(25℃) |
| Растворимость в воде | very soluble H2O [HAW93] |
| Пределы воздействия | Ceiling 0.1 ppm vapor (ACGIH). |
| Стабильность | Unstable. Violently explosive in the concentrated or pure states. Readily forms explosive compounds with heavy metals. This is a dangerous and hard to handle substance which must not be prepared or handled by non-experts. |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | 6P5C4D5D7I |
| Система регистрации веществ EPA | Hydrazoic acid (7782-79-8) |
| РИДАДР | 0473 |
| Класс опасности | 1.1A |
| Банк данных об опасных веществах | 7782-79-8(Hazardous Substances Data) |
| Токсичность | LD50 in mice (mg/kg): 21.5 i.p. (Graham) |
Hydrazoic acid химические свойства, назначение, производство
Описание
Hydrazoic acid or hydrogen azide is a dangerous explosion risk when shocked or heated. It is the gas-forming agent in many air bag systems in automobiles and escape chutes in airplanes.Химические свойства
Colorless, volatile liquid; obnoxious odor.Soluble in water.Физические свойства
Colorless, volatile liquid; pungent disagreeable odor; density 1.09 g/mL;solidifies at -80°C; boils at 37°C; highly soluble in water; soluble in alkalies,alcohol and ether; pKa4.6 at 25°C.Использование
Hydrazoic acid is used in making heavymetal azides for detonators. It forms readilywhen sodium azide reacts with acid orhydrazine is mixed with nitrous acid.Определение
A colorless liquid with a nauseating smell. It is highly poisonous and explodes in the presence of oxygen and oxidizing agents. It can be made by distilling a mixture of sodium azide (NaN3) and a dilute acid. It is usually used as an aqueous solution. The salts of hydrazoic acid (azides), especially lead azide (Pb(N3)2), are used in detonators because of their ability to explode when given a mechanical shock.Методы производства
Hydrazoic acid is formed (1) by reaction of sodium nitrate with molten sodamide, (2) by reaction of nitrous oxide with molten sodamide, (3) by reaction of nitrous acid and hydrazinium ion (N2H5 + ), (4) by oxidation of hydrazinium salts, (5) by reaction of ethyl nitrite with NaOH solution and acidifying.Подготовка
Hydrazoic acid is prepared by reacting sulfuric acid with sodium azide:H2SO4 + NaN3 → HN3 + Na2SO4
or by treating hydrazine with nitrous acid:
N2H4 + HNO2 → HN3 + 2H2O
or by heating sodium amide with nitrous oxide:
NaNH2 + N2O → HN3 + NaOH
Реакции
Hydrazoic acid reacts (1) with metals, e.g., magnesium, aluminum, zinc, iron, to form azides or hydrazoates (or trinitrides), (2) with heavy metal salt solutions to form insoluble azides, e.g., silver azide AgN3, mercury(I) azide HgN3, lead azide PbN6. Silver, mercury(I), and copper(I) azides decompose in the light to form nitrogen plus the metal. (3) It reacts with NH4OH to form ammonium azide NH4·N3, (4) with hydrazine to form hydrazine azide N2H4·HN3, (5) with sodium hypochlorite plus acetic acid to form chlorazide ClN3, explosive, (6) with sodium amalgam to form NH3 with some hydrazine, (7) with potassium permanganate to form nitrogen and H2O.Опасность
Dangerous explosion risk when shocked or heated. Strong irritant to eyes and mucous membranes.Угроза здоровью
The acute toxicity of hydrazoic acidthrough inhalation and other routes of exposurehas been found to be high to very high.The symptoms and the intensity of poisoningare similar to sodium azide. It is, however,less toxic than hydrogen cyanide. Inhumans, inhalation of its vapors can produceirritation of eyes and respiratory tract, bronchitis,headache, dizziness, weakness, anddecreased blood pressure (Matheson 1983).Prolonged exposure to high concentrationscan result in collapse, convulsion, and death.An exposure to 1100 ppm for 1 hour waslethal to rats. Chronic exposure to a lowlevel of this compound in air may producehypotension.Animals given intraperitoneal dosages ofhydrazoic acid showed the symptoms ofheavy breathing, convulsions, depression,and fall in blood pressure. It affected thecentral nervous system, but no damage wasobserved in the liver or kidney.
LD50 value, intraperitoneal (mice): 22 mg/kg.
Пожароопасность
In pure form or highly concentrated solution, hydrazoic acid is a dangerous explosive compound. It is unstable and sensitive to heat and shock. The explosion hazard decreases significantly with more dilute solutions.It forms shock-sensitive metal azides when react with metal salts, and fluorine azide with fluorine (Lawless and Smith 1968) and susceptible to form chlorine azide and bromine azide with chlorine gas and bromine vapor. All these products can explode violently on impact. With carbon disulfide it forms a violently explosive salt (Mellor 1946; NFPA 1997).
Утилизация отходов
Hydrazoic acid may be destroyed by convertingit to sodium azide. The latter isdecomposed with nitrous acid in a hood(National Research Council 1995). The followingmethod is used. It is diluted in waterto a strength below 5%; or its solution inorganic solvents that is immiscible in wateris shaken vigorously with water in a separatoryfunnel. The aqueous solution containinghydrazoic acid is neutralized with sodiumhydroxide and separated from any organiclayer. Sodium azide, so formed, is destroyedby reacting the aqueous solution with anexcess of sodium nitrite followed by 20%sulfuric acid until the solution is acidic. Thereaction is carried out in a three-necked flaskequipped with a stirrer, a dropping funnel,and a gas outlet line to vent out nitric oxide.The reaction mixture is flushed down thedrain.Hydrazoic acid запасные части и сырье
Hydrazoic acid поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| 18871490254 | CHINA | 28172 | 58 | |
| +86-029-89586680 +86-18192503167 |
China | 7724 | 58 | |
| +86-0592-6210733 | China | 32343 | 55 | |
| 029-029-81148696 18161915376 |
China | 9997 | 58 |