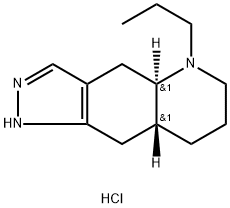
(?)-Quinpirole hydrochloride
- английское имя(-)-QUINPIROLE HYDROCHLORIDE
- CAS №85798-08-9
- CBNumberCB4687090
- ФормулаC13H22ClN3
- мольный вес255.79
- номер MDLMFCD00069336
- файл Mol85798-08-9.mol
химическое свойство
| температура хранения | -20°C |
| растворимость | 0.1 M HCl: soluble23mg/mL |
| форма | solid |
| цвет | white |
| оптическая активность | [α]25/D 124.5°, c = 0.4 in H2O(lit.) |
| Растворимость в воде | water: 7.3mg/mL |
| Стабильность | Hygroscopic |
| FDA UNII | T6I2W5V2K1 |
| UNSPSC Code | 12352200 |
| NACRES | NA.77 |
(?)-Quinpirole hydrochloride химические свойства, назначение, производство
Использование
(-)-Quinpirole hydrochloride has been used as a selective D2 dopamine (DA) receptor agonist in various experiments.Биохимия/физиол Действия
Quinpirole is a dopamine agonist with high affinity for the D2 and D3 dopamine receptor subtypes. Specific [3H]quinpirole binding in rat brain was saturable, and dependent on temperature, membrane concentration, sodium concentration and guanine nucleotides. The putative D2 dopamine receptor agonist quinpirole (LY 171,555) is the most widely used D2 agonist in in vivo and in vitro studies. Quinpirole hydrochloride is an active enantiomer of (±)-quinpirole.Saturation analysis revealed high affinity binding characteristics (KD = 2.3 +/- 0.3 nM) which were confirmed by association-dissociation kinetics. The regional distribution of [3H]quinpirole binding sites roughly paralleled the distribution of [3H]spiperone binding sites, with greatest densities present in the striatum, nucleus accumbens and olfactory tubercles. A variety of drugs, most notably monoamine oxidase inhibitors (MAOls), inhibit the binding of [3H]quinpirole, but not [3H]spiperone or [3H](-)N-n-Propylnorapomorphine, in rat striatal membranes by a mechanism that does not appear to involve the enzymatic activity of MAO. Clinically antidepressant MAOIs exhibited selectivity between sites labeled by [3H]quinpirole and [3H]spiperone as did a number of structurally related propargylamines and N-acylethylenediamine derivatives and other drugs such as debrisoquin and phenylbiguanide. The MAOIs clorgyline and Ro 41-1049 were the most potent. MAOIs interact with a novel binding site that is labeled by [3H]quinpirole or that modulates [3H]quinpirole binding. This site may be associated with D2-like dopamine receptors.(?)-Quinpirole hydrochloride поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +1-781-999-5354 +1-00000000000 |
United States | 32161 | 58 | |
| +1-708-310-1919 +1-13798911105 |
United States | 6391 | 58 | |
| United States | 38631 | 58 | ||
| +8618327326525 | China | 8467 | 58 | |
| +1-+1(833)-552-7181 | United States | 52924 | 58 | |
| 821-50328103-801 18930552037 |
China | 15839 | 69 | |
| 021-61415566 800-8193336 |
China | 51456 | 80 | |
| 888-539-0666 | United States | 8447 | 60 | |
| 021-65675885 18964387627 |
China | 9804 | 58 | |
| 010-010-010-62971590 18548936886 |
China | 6897 | 58 |