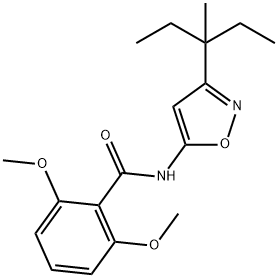
ISOXABEN
- русский язык имя
- английское имяISOXABEN
- CAS №82558-50-7
- CBNumberCB4362249
- ФормулаC18H24N2O4
- мольный вес332.39
- EINECS407-190-8
- номер MDLMFCD00072433
- файл Mol82558-50-7.mol
химическое свойство
| Температура плавления | 175-179 °C |
| Температура кипения | 469.52°C (rough estimate) |
| плотность | 1.2149 (rough estimate) |
| показатель преломления | 1.5700 (estimate) |
| температура хранения | 0-6°C |
| пка | 11.44±0.70(Predicted) |
| FDA UNII | 101V41EEA4 |
| Система регистрации веществ EPA | Isoxaben (82558-50-7) |
| Пестициды Закон о свободе информации (FOIA) | Isoxaben |
| UNSPSC Code | 41116107 |
| NACRES | NA.24 |
| Коды опасности | Xn |
| Заявления о рисках | 53-40 |
| Заявления о безопасности | 61-36 |
| WGK Германия | 3 |
| RTECS | CV4370300 |
| Банк данных об опасных веществах | 82558-50-7(Hazardous Substances Data) |
ISOXABEN химические свойства, назначение, производство
Использование
Isoxaben is used primarily for preemergence control of annual broadleaf weeds. Isoxaben is usually applied to soil either with light incorporation or before application of water (at least 0.5 in) within 3 weeks. As is the case with DCB, it is most effective on weed seedlings before emergence.Определение
ChEBI: A benzamide obtained by formal condensation of the carboxy group of 2,6-dimethoxybenzoic acid and the amino group of 3-(3-methylpentan-3-yl)-1,2-oxazol-5-amine.Фармаколо?гия
Although isoxaben is readily absorbed through the root system, foliar absorption and translocation is poor. Reduced root absorption may be partly responsible for the tolerance of some dicot species to isoxaben, although differences in the site of action appears to be the major contributing factor in tolerance (15).Up to 50% of absorbed isoxaben is metabolized within 6 days following root application (16). Differences in metabolism cannot explain the selectivity of isoxaben between tolerant and susceptible species (17,18). Metabolism of isoxaben involves hydroxylation of the propyl side group and glucosylation. 2,6-dimethoxybenzamide is found as a minor metabolite (19). Isoxaben prevents the germination and growth of seedlings before emergence, by inhibiting cell division.The primary mode of action of isoxaben is inhibition of cellulose biosynthesis, although the exact mechanism is unclear. Isoxaben has been shown to specifically inhibit the incorporation of glucose into the acid-insoluble fraction (presumed to be cellulose) of the cell walls of Arabidopsis thaliana (20) and soybean cell suspension cultures (21). This herbicide also disrupts cell plate formation in root tips (22) and tobacco suspension cells (23). Isoxaben affects a different site of action compared with DCB, as it inhibits cytokinesis at an earlier stage in which callose is deposited at the developing cell plate (23). There are no known cases of resistance to this herbicide.
Метаболизм
Isoxaben is adsorbed strongly to soil and therefore has very limited mobility. Volatilization and photodegradation of isoxaben is negligible when applied to soil. Isoxaben is mainly degraded by soil organisms and has an average half-life of 1 to 2 months in the field, providing an average of 5 to 6 months of weed control at normal rates of application (19).ISOXABEN поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| 18017610038 | CHINA | 3619 | 58 | |
| 18871490254 | CHINA | 28172 | 58 | |
| +86-023-6139-8061 +86-86-13650506873 |
China | 39894 | 58 | |
| +1-781-999-5354 +1-00000000000 |
United States | 32161 | 58 | |
| +86-0371-86658258 +8613203830695 |
China | 30233 | 58 | |
| United States | 38631 | 58 | ||
| +86-0512-83500002 +8615195660023 |
China | 23046 | 58 | |
| +86-18621343501; +undefined18621343501 |
China | 33338 | 58 | |
| +1-+1(833)-552-7181 | United States | 52924 | 58 | |
| +8613817748580 | China | 40066 | 58 |