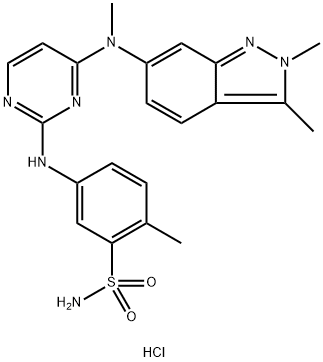
Pazopanib Hydrochloride
- русский язык имя
- английское имяPazopanib Hydrochloride
- CAS №635702-64-6
- CBNumberCB42495435
- ФормулаC21H24ClN7O2S
- мольный вес473.98
- EINECS619-728-0
- номер MDLMFCD12546138
- файл Mol635702-64-6.mol
| Температура плавления | >290°C (dec.) |
| температура хранения | Hygroscopic, Refrigerator, under inert atmosphere |
| растворимость | Acetonitrile (Slightly), DMSO (Slightly) |
| форма | Yellow powder. |
| цвет | White to Off-White |
| Стабильность | Hygroscopic |
| ИнЧИКей | MQHIQUBXFFAOMK-UHFFFAOYSA-N |
| SMILES | CC1N(N=C2C=C(N(C3C=CN=C(NC4C=CC(C)=C(S(=O)(=O)N)C=4)N=3)C)C=CC=12)C.Cl |
| Словарь онкологических терминов NCI | pazopanib hydrochloride |
| FDA UNII | 33Y9ANM545 |
| Словарь наркотиков NCI | pazopanib hydrochloride |
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H315:При попадании на кожу вызывает раздражение.
H319:При попадании в глаза вызывает выраженное раздражение.
H335:Может вызывать раздражение верхних дыхательных путей.
H302:Вредно при проглатывании.
H332:Вредно при вдыхании.
-
оператор предупредительных мер
P261:Избегать вдыхания пыли/ дыма/ газа/ тумана/ паров/ аэрозолей.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
Pazopanib Hydrochloride химические свойства, назначение, производство
Описание
Pazopanib Hydrochloride is the hydrochloride salt of a small molecule inhibitor of multiple protein tyrosine kinases with potential antineoplastic activity. It is an oral second-generation multitarget TKI developed by GSK and approved for marketing by the FDA in 2009 and the EMA in 2010. It targets the VEGFR, platelet-derived growth factor receptor, and c-kit, key proteins responsible for tumor growth and survival. It is used to treat patients with advanced RCC and advanced soft tissue sarcoma who have experienced chemotherapy. Pazopanib Hydrochloride has a role as an antineoplastic agent, a vascular endothelial growth factor receptor antagonist, a tyrosine kinase inhibitor, and an angiogenesis-modulating agent.Использование
Pazopanib Hydrochloride (GW786034, Votrient, Armala) is a novel multi-target inhibitor of VEGFR1, VEGFR2, VEGFR3, PDGFR, FGFR, c-Kit and c-Fms with IC50 of 10 nM, 30 nM, 47 nM, 84 nM, 74 nM, 140 nM and 146 nM, respectively.Определение
ChEBI: A hydrochloride salt prepared from equimolar amounts of pazopanib and hydrochloric acid. Used for treatment of kidney cancer.Клиническое использование
Pazopanib is a potent and selective multi-targeted receptor tyrosine kinase inhibitor of VEGFR-1, VEGFR-2, VEGFR-3, PDGFR-a/b, and c-kit that blocks tumor growth and inhibits angiogenesis. It was approved for renal cell carcinoma by the U.S. Food and Drug Administration in 2009 and is marketed under the trade name Votrient by the drug’s manufacturer, GlaxoSmithKline.Побочные эффекты
Pazopanib is synthesized in five chemical steps starting from 3-methyl-6-nitroindazole, which is converted to the corresponding 2,3-dimethylindazole analog via N-methylation with trimethyloxonium tetrafluoroborate. Subsequent reduction of the nitro group to the amino group using tin chloride followed by condensation with 2,4dichloropyrimidine yields a chloropyrimidinylaminoindazole intermediate. The final two steps leading up to pazopanib consist of an N-methylation reaction using iodomethane and cesium carbonate followed by condensation with 5-amino-2-methylbenzenesulfonamide.использованная литература
[1] Sodeifian, G. et al. “Solubility of pazopanib hydrochloride (PZH, anticancer drug) in supercritical CO2: Experimental and thermodynamic modeling.” The Journal of Supercritical Fluids 55 1 (2022): 0.[2] “Stability Indicating HPTLC Method Development and Validation for the Estimation of Pazopanib Hydrochloride in Bulk and its Dosage Form.” International Journal of Pharmaceutical Research 18 1 (2020).
[3] K. Kawasaki . “Retrospective Safety Analysis in Advanced Soft Tissue Sarcoma Patients of Pazopanib Hydrochloride.” Annals of Oncology 24 (2013): Page ix38.
[4] Gupta, Amit and Rashmi Dahima. “Application of Simplex Lattice Mixture design and desirability function in the development and Optimization of SEDDS for protein kinase inhibitor-Pazopanib Hydrochloride.” Research Journal of Pharmacy and Technology 83 1 (2023): 0.
Pazopanib Hydrochloride запасные части и сырье
Pazopanib Hydrochloride поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +86-0571-85134551 | China | 15352 | 58 | ||
| 01056380788-8515; +8618518759099 |
China | 52 | 58 | ||
| +86-18752526868 | China | 81 | 58 | ||
| +86-18600796368 +86-18600796368 |
China | 484 | 58 | ||
| 010-60279497 | CHINA | 1803 | 55 | ||
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | ||
| 008657128800458; +8615858145714 |
China | 7782 | 55 | ||
| +undefined-21-51877795 | China | 32965 | 60 | ||
| 15380796838 | CHINA | 340 | 58 | ||
| 15950718863 | CHINA | 295 | 58 |