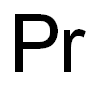
празеодимий
- английское имяPRASEODYMIUM
- CAS №7440-10-0
- CBNumberCB4156796
- ФормулаPr
- мольный вес140.91
- EINECS231-120-3
- номер MDLMFCD00011174
- файл Mol7440-10-0.mol
| Температура плавления | 931 °C (lit.) |
| Температура кипения | 3520 °C (lit.) |
| плотность | 6.71 g/mL at 25 °C (lit.) |
| температура хранения | Flammables area |
| форма | powder |
| цвет | White |
| Удельный вес | 6.782 |
| РН | <1 (20°C in H2O) |
| удельное сопротивление | 68 μΩ-cm, 20°C |
| Растворимость в воде | Reacts with water. |
| Чувствительный | Air & Moisture Sensitive |
| Мерк | 13,7797 |
| Пределы воздействия | ACGIH: TWA 2 ppm; STEL 4 ppm OSHA: TWA 2 ppm(5 mg/m3) NIOSH: IDLH 25 ppm; TWA 2 ppm(5 mg/m3); STEL 4 ppm(10 mg/m3) |
| Справочник по базе данных CAS | 7440-10-0(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | NKN7EZA750 |
| Система регистрации веществ EPA | Praseodymium (7440-10-0) |
| UNSPSC Code | 41116107 |
| NACRES | NA.24 |
| Коды опасности | F,C | |||||||||
| Заявления о рисках | 17-11-34 | |||||||||
| Заявления о безопасности | 17-7/9-33-16-45-36/37/39-27-26-23 | |||||||||
| РИДАДР | UN 3208 4.3/PG 1 | |||||||||
| WGK Германия | 3 | |||||||||
| F | 1-10 | |||||||||
| TSCA | Yes | |||||||||
| Класс опасности | 8 | |||||||||
| Группа упаковки | III | |||||||||
| кода HS | 28053090 | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
опасность
-
вредная бумага
H315:При попадании на кожу вызывает раздражение.
H319:При попадании в глаза вызывает выраженное раздражение.
H413:Может вызвать долгосрочные отрицательные последствия для водных организмов.
H250:Спонтанно воспламеняется на воздухе.
-
оператор предупредительных мер
P210:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P231+P232:Обращаться с продуктом и хранить его в атмосфере инертного газа . Беречь от влаги.
P273:Избегать попадания в окружающую среду.
P302+P352:ПРИ ПОПАДАНИИ НА КОЖУ: Промыть большим количеством воды.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
P370+P378:При пожаре тушить сухим песком, сухим химическим порошком или спиртостойкой пеной.
празеодимий химические свойства, назначение, производство
Химические свойства
grey powderФизические свойства
Praseodymium is a silvery-white, soft metal that is easily formed into various shapes. Whenthe pure metal is exposed to the air, a green oxide coating forms on its surface. To preventoxidation, praseodymium is usually kept in oil in a covered container.Its melting point is 931°C, its boiling point is 3,520°C, and its density is 6.77g/cm3.
Изотопы
There are 45 isotopes of praseodymium. All are artificially produced and radioactivewith half-lives ranging from several hundred nanoseconds to 23.6 days. Only oneis stable (Pa-141), and it makes up 100% of the praseodymium found in the Earth’scrust.Происхождение имени
The name is derived from two Greek words, prasios and didymos, which together mean “green twins.”Вхождение
Praseodymium is the 41st most abundant element on Earth and is found in the ores of monazite,cerite, bastnasite, and allanite along with other rare-earths. Praseodymium is also the stableisotope resulting from the process of fission of some other heavy elements, such as uranium.Praseodymium is mainly found in monazite sands and bastnasite ores. The monazite sandscontain all of the rare-earths and are found in river sand in India and Brazil as well as inFlorida beach sand. A large deposit of bastnasite exists in California.
Praseodymium is separated from its ore and other rare-earths by a process called ionexchange, which exchanges one type of ion for another.
Характеристики
As a metal, Pr is hygroscopic (adsorbs water) and tarnishes in the atmosphere. It will reactwith water to liberate hydrogen. It is soluble in acids and forms greenish salts, along with someother rare-earths. It is used to fabricate the electrodes for high-intensity lights.История
In 1879, Lecoq de Boisbaudran isolated a new earth, samaria, from didymia obtained from the mineral samarskite. Six years later, in 1885, von Welsbach separated didymia into two others, praseodymia and neodymia, which gave salts of different colors. As with other rare earths, compounds of these elements in solution have distinctive sharp spectral absorption bands or lines, some of which are only a few Angstroms wide. Praseodymium occurs along with other rare-earth elements in a variety of minerals. Monazite and bastnasite are the two principal commercial sources of the rare-earth metals. Ion-exchange and solvent extraction techniques have led to much easier isolation of the rare earths and the cost has dropped greatly. Thirty-seven isotopes and isomers are now recognized. Praseodymium can be prepared by several methods, such as by calcium reduction of the anhydrous chloride or fluoride. Misch metal, used in making cigarette lighters, contains about 5% praseodymium metal. Praseodymium is soft, silvery, malleable, and ductile. It was prepared in relatively pure form in 1931. It is somewhat more resistant to corrosion in air than europium, lanthanum, cerium, or neodymium, but it does develop a green oxide coating that splits off when exposed to air. As with other rare-earth metals it should be kept under a light mineral oil or sealed in plastic. The rare-earth oxides, including Pr2O3, are among the most refractory substances known. Along with other rare earths, it is widely used as a core material for carbon arcs used by the motion picture industry for studio lighting and projection. Salts of praseodymium are used to color glasses and enamels; when mixed with certain other materials, praseodymium produces an intense and unusually clean yellow color in glass. Didymium glass, of which praseodymium is a component, is a colorant for welder’s goggles. The metal (99.9% pure) is priced at about $4/g.Использование
Praseodymium salts, ingredient of mischmetal, core material for carbon arcs, colorant in glazes and glasses, catalyst, phosphors, lasers.Подготовка
Praesodymium may be recovered from its minerals monazite and bastanasite. The didymia extract of rare earth minerals is a mixture of praesodymia and neodymia, primarily oxides of praesodymium and neodymium. Several methods are known for isolation of rare earths. These are applicable to all rare earths including praesodymium. They include solvent extractions,ionexchange, and fractional crystallization. While the first two methods form easy and rapid separation of rare earth metals, fractional crystallization is more tedious. Extractions and separations of rare earths have been discussed in detail earlier (see Neodymium and Cerium).Praesodymium metal can be obtained from its anhydrous halides by reduction with calcium. The metal also may be prepared by electrolysis of fused praesodymium chloride at elevated temperatures (about 1,000°C).Alternatively, an eutectic mixture of praesodymium chloride, potassium chloride, and sodium chloride may be electrolyzed. In such electrolysis graphite is the anode and tungsten the cathode.
Определение
A soft ductile malleable silvery element of the lanthanoid series of metals. It occurs in association with other lanthanoids. Praseodymium is used in several alloys, as a catalyst, and in enamel and yellow glass for eye protection. Symbol: Pr; m.p. 931°C; b.p. 3512°C; r.d. 6.773 (20°C); p.n. 59; r.a.m. 140.91.Опасность
If praseodymium gets wet or is submerged in water, the hydrogen released may explode. Itmust be kept dry and protected from the atmosphere.празеодимий запасные части и сырье
запасной предмет
празеодимий поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| 18871490254 | CHINA | 28172 | 58 | |
| +86-0551-65418671 +8618949823763 |
China | 34563 | 58 | |
| +86-029-89586680 +86-18192503167 |
China | 7724 | 58 | |
| +86-86-13583358881 +8618560316533 |
China | 3094 | 58 | |
| 571-88938639 +8617705817739 |
China | 52849 | 58 | |
| 02120970332; +8613524231522 |
China | 2904 | 58 | |
| China | 12341 | 58 | ||
| United States | 24072 | 58 | ||
| +86-852-30606658 | China | 24727 | 58 | |
| +86-85511178; +86-85511178; |
China | 35425 | 58 |