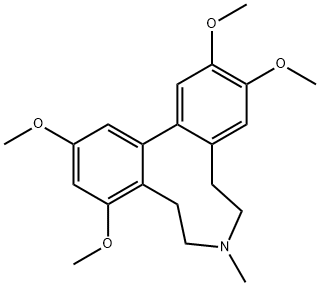
протостефанин
- английское имяprotostephanine
- CAS №549-28-0
- CBNumberCB41481015
- ФормулаC21H27NO4
- мольный вес357.44
- файл Mol549-28-0.mol
химическое свойство
| Температура плавления | 84-86° (Pecherer); mp 75° (Kondo) |
| Температура кипения | 518.5±50.0 °C(Predicted) |
| плотность | 1.095±0.06 g/cm3(Predicted) |
| пка | 8.75±0.20(Predicted) |
| FDA UNII | 8HEL0402TN |
протостефанин химические свойства, назначение, производство
Описание
A Stephania alkaloid, this base is found in S. japonica Miers. It forms colourless prisms from MeOH which contain solvent of crystallization, m.p. 75°C. The solvent may be removed under vacuum when the dry alkaloid has a higher melting point of 90-S°C. It is optically inactive and yields crystalline saits, e.g. the hydrochloride, m.p. 150°C (dec.); platinichloride, orange prisms, m.p. 223°C (dec.); methiodide, m.p. 220-loC and the methomethylsulphate, m.p. 235°C after sintering at 227°C. The structure has recently been confirmed by a synthesis which follows a route related to the biosynthetic pathway.использованная литература
Kondo, Watanabe., J. Pharrn. Soc., Japan, 58,46 (1938)Kondo, Takeda., Chern. Abstr., 49, 1076 (1955)
Synthesis: Battersby et al., Chern. Cornrnun., 20, 1214 (1968)