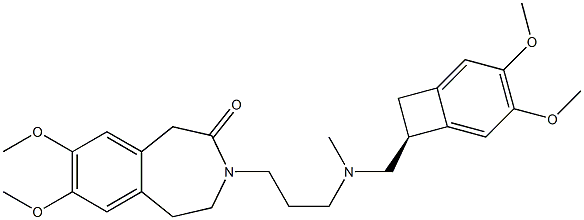
Ивабрадин
- английское имяIvabradine
- CAS №155974-00-8
- CBNumberCB41178988
- ФормулаC27H36N2O5
- мольный вес468.59
- номер MDLMFCD04975447
- файл Mol155974-00-8.mol
химическое свойство
| Температура кипения | 626.9±55.0 °C(Predicted) |
| плотность | 1.146±0.06 g/cm3(Predicted) |
| пка | 8.77±0.50(Predicted) |
| FDA UNII | 3H48L0LPZQ |
| Код УВД | C01EB17 |
Ивабрадин химические свойства, назначение, производство
Описание
Ivabradine is a first selective and specific If inhibitor that was approved by EMEA in November for symptomatic treatment of chronic stable angina pectoris in patients with normal sinus rhythm. This is the first agent to lower heart rate by inhibiting the cardiac pacemaker If current. The compound was discovered and developed by Servier and is currently being marketed in Ireland.Определение
ChEBI: A member of the class of benzazepines that is 7,8-dimethoxy-1,3,4,5-tetrahydro-3-benzazepin-2-one in which the amide hydrogen is replaced by a [{[(7S)-3,4-dimethoxybicyclo[4.2.0]octa-1,3,5-trien-7-yl]methyl}(methyl)amino]propyl} group. Use (as its hydrochloride salt) to treat patients with angina who have intolerance to beta blockers and/or heart failure.Синтез
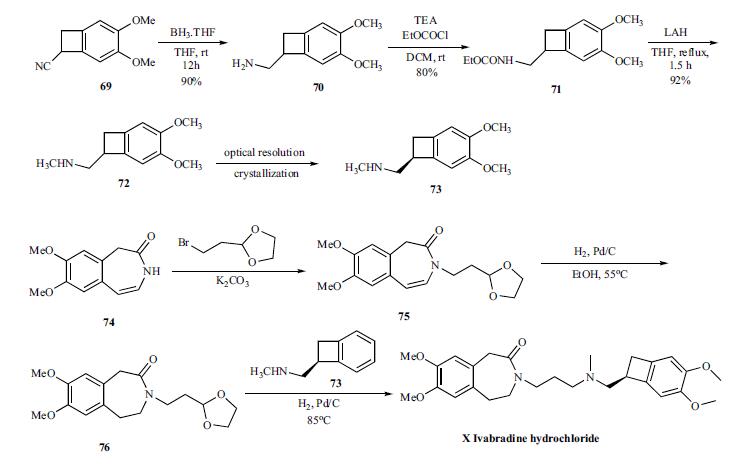
The convergent synthesis of ivabradine was accomplished by coupling the key benzocylclobutanyl amine 73 with oxadioxalane 76 in an in situ deprotection and amination as shown in Scheme 13. For the synthesis of the key amine 73, cyano group of compound 69 is reduced with borane-THF to give amine 70 in 90% yield, which was reacted with ethyl chloroformate to give carbamate 71 in 80% yield. Complete reduction of the carbamate was accomplished by refluxing with LAH in THF to give racemic methyl amine 72 in 92% yield, which was then resolved by crystallizing with N-acetyl ¨CL-glutamic acid to give chiral salt 73. Prior to the next step, the amine is converted to the hydrochloride salt.The coupling partner 76 to make ivabradine was prepared from the azepinone 74 by first reacting with bromoethyldioxalane to give 75. The olefin in 75 was reduced by hydrogenating with palladium/carbon catalyst at 55??C to give 76. To the same pot, the amine 73 was added and hydrogenated to give reductive amination product ivabradine hydrochloride (X) in very good yields.
Ивабрадин запасные части и сырье
Ивабрадин поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 | |
| 18871490254 | CHINA | 28172 | 58 | |
| +86-023-6139-8061 +86-86-13650506873 |
China | 39894 | 58 | |
| +86-13806087780 | China | 17365 | 58 | |
| +8615255079626 | China | 23541 | 58 | |
| 029-89275612 +8618991951683 |
China | 2251 | 58 | |
| +86-029-89586680 +86-18192503167 |
China | 7724 | 58 | |
| +86-86-13583358881 +8618560316533 |
China | 3094 | 58 | |
| +8613373514458 | China | 203 | 58 |