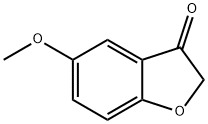
5-Methoxybenzofuran-3(2H)-one
- русский язык имя
- английское имя5-Methoxybenzofuran-3(2H)-one
- CAS №39581-55-0
- CBNumberCB3840863
- ФормулаC9H8O3
- мольный вес164.16
- номер MDLMFCD08544410
- файл Mol39581-55-0.mol
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H315:При попадании на кожу вызывает раздражение.
H319:При попадании в глаза вызывает выраженное раздражение.
H335:Может вызывать раздражение верхних дыхательных путей.
H302:Вредно при проглатывании.
-
оператор предупредительных мер
P261:Избегать вдыхания пыли/ дыма/ газа/ тумана/ паров/ аэрозолей.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
5-Methoxybenzofuran-3(2H)-one химические свойства, назначение, производство
Синтез
5-Methoxybenzofuran-3(2H)-one is synthesised using 2-chloro-1-(2-hydroxy-5-methoxyphenyl)ethanone as raw material by chemical reaction. The specific synthesis steps are as follows:To an ice-cooled solution of boron trichloride (1.2 equiv., 10 mmol, 10 ml of a solution of 1M BC13 in dichloromethane) under N2-atmosphere was added dropwise a solution of J (1 g, 8 mmol,) in dichloromethane (5 ml). Then, chloroacetonitrile (0.7 g, 10 mmol, 1.2 equiv. ) was added dropwise, followed by aluminum(III) chloride (0.5 g, 4 mmol, 0.5 equiv. ) in one portion. The reaction mixture was allowed to warm up to room temperature and was stirred for 6 h. The reaction mixture was diluted with dichloromethane and quenched with IN hydrochloric acid at 0°C. After stirring for 10 min, the aqueous layer was extracted with dichloromethane. The combined organic layers were washed with brine, dried (MgS04) and concentrated Purification by flash chromatography on silica gel (eluent: dichloromethane) afforded the desired product K (1 g, yield = 60percent). Intermediate K (0.15 g, 0.75 mmol, I equiv. ) and potassium acetate (0.22 g, 2.2 mmol, 3 equiv. ) were refluxed in ethanol (10 ml) for 1 h. After cooling, the reaction mixture was filtered and concentrated. The residue was mixed with water, and acidified with IN hydrochloric acid to pH = 1. The aqueous solution was extracted with ethyl acetate, the combined extracts were dried (MgS04), filtered and concentrated to afford the desired product L (110 mg, yield = 90 percent) as a pink solid The experimental procedures for the condensation reaction of compound L with 4-nitroaniline in acetic acid to form compound M, followed by Vilsmeier-Haack formulation and subsequent Knoevenagel condensation of the benzofuran carbaldehyde N with ethyl cyanoacetate to form compound 0, and finally, intramolecular cyclisation to compound 37 were performed using analogous procedures as exemplified in example 1 for the synthesis of compound f starting from compound a. Compound 37 was obtained as a yellow powder (30 mg, purity (LC) = 80 percent).

5-Methoxybenzofuran-3(2H)-one запасные части и сырье
5-Methoxybenzofuran-3(2H)-one поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86-(0)57185586718 +86-13336195806 |
China | 29792 | 60 | |
| +86-755-89396905 +86-15013857715 |
China | 10453 | 58 | |
| 0519-83990708 | CHINA | 498 | 58 | |
| 8485655694 | United States | 63687 | 58 | |
| 15705188088 | CHINA | 1268 | 58 | |
| +8618523575427 | China | 49732 | 58 | |
| +86-021-67759270 +8617717059219 |
China | 1030 | 58 | |
| +86-0371-86658258 +8613203830695 |
China | 30233 | 58 | |
| +86-0551-65418671 +8618949823763 |
China | 34563 | 58 | |
| +8615303909093 | China | 2914 | 58 |