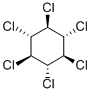
БЕТА-HCH
- английское имяBETA-HCH
- CAS №319-85-7
- CBNumberCB3676013
- ФормулаC6H6Cl6
- мольный вес290.83
- EINECS206-271-3
- номер MDLMFCD00135948
- файл Mol319-85-7.mol
| Температура плавления | ≥300 °C |
| Температура кипения | 373.64°C (rough estimate) |
| плотность | 1.9 g/cm3 |
| давление пара | 4.66 at 25 °C (Banerjee et al., 1990) |
| показатель преломления | 1.630 (589.3 nm) |
| Fp | 11 °C |
| температура хранения | APPROX 4°C |
| растворимость | Soluble in ethanol, benzene, and chloroform (Weast, 1986) |
| форма | Solid |
| Растворимость в воде | 0.24mg/L(25 ºC) |
| БРН | 1907338 |
| констант закона Генри | 0.53 at 5 °C, 0.91 at 10 °C, 2.17 at 20 °C, 5.23 at 30 °C, 8.68 at 35 °C:in 3% NaCl solution: 1.09 at 5 °C, 1.48 at 15 °C, 2.76 at 25 °C, 4.24 at 35 °C (dynamic headspace-GC, Sahsuvar et al., 2003 |
| Рейтинг продуктов питания EWG | 2 |
| FDA UNII | YM80ODM9PD |
| Система регистрации веществ EPA | .beta.-Hexachlorocyclohexane (319-85-7) |
| UNSPSC Code | 41116107 |
| NACRES | NA.24 |
| Коды опасности | F,T,N,Xi | |||||||||
| Заявления о рисках | 11-23/24/25-39/23/24/25-50/53-40-25-21-67-66-52/53-36 | |||||||||
| Заявления о безопасности | 7-16-24-45-36/37-61-60-22-26 | |||||||||
| РИДАДР | UN1230 3/PG 2 | |||||||||
| WGK Германия | 3 | |||||||||
| RTECS | GV4375000 | |||||||||
| Класс опасности | 6.1 | |||||||||
| Группа упаковки | III | |||||||||
| Банк данных об опасных веществах | 319-85-7(Hazardous Substances Data) | |||||||||
| Токсичность | Acute oral LD50 for rats 6,000 mg/kg (RTECS, 1985). | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)



-
сигнальный язык
опасность
-
вредная бумага
H301:Токсично при проглатывании.
H351:Предполагается, что данное вещество вызывает раковые заболевания.
H410:Чрезвычайно токсично для водных организмов с долгосрочными последствиями.
H312:Вредно при попадании на кожу.
-
оператор предупредительных мер
P202:Перед использованием ознакомиться с инструкциями по технике безопасности.
P264:После работы тщательно вымыть кожу.
P273:Избегать попадания в окружающую среду.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P301+P310:ПРИ ПРОГЛАТЫВАНИИ: Немедленно обратиться за медицинской помощью. Прополоскать рот.
P302+P352+P312:ПРИ ПОПАДАНИИ НА КОЖУ: Промыть большим количеством воды. Обратиться за медицинской помощью при плохом самочувствии.
БЕТА-HCH химические свойства, назначение, производство
Химические свойства
BHC is a white-to-brownish crystalline solid with a musty, phosgene-like odor.Физические свойства
Although β-BHC is a solid at room temperature, the odor threshold concentration is 0.32 μg/kg (Sigworth, 1964).Использование
β-1,2,3,4,5,6-Hexachlorocyclohexane is an organochloride which is one of the isomers of hexachlorocyclohexane and is also an byproduct of insecticide Lindane (L465990).Определение
ChEBI: The beta-isomer of hexachlorocyclohexane.Общее описание
Colorless crystals. Corrosive to aluminum and many other metals.Реакции воздуха и воды
Slightly soluble in water.Профиль реактивности
Halogenated aliphatic compounds, such as BETA-HCH, are moderately or very reactive. Reactivity generally decreases with increased degree of substitution of halogen for hydrogen atoms. Materials in this group may be incompatible with strong oxidizing and reducing agents. Also, they may be incompatible with many amines, nitrides, azo/diazo compounds, alkali metals, and epoxides. Dehydrochlorination occurs in the presence of alkalis.Угроза здоровью
ACUTE/CHRONIC HAZARDS: Highly toxic carcinogen. May cause irritation on contact. Hazardous decomposition products.Профиль безопасности
Confirmed carcinogen with experimental neoplastigenic data. Mildly toxic by ingestion. When heated to decomposition it emits very toxic fumes of Cl-, HCl, and phosgene. See also BENZENE HEXACHLORIDE and other benzenehexachloride entries.Возможный контакт
The major commercial usage of BHC is based upon its insecticidal properties. α-BCH is used as an Agricultural chemical, pesticide, pharmaceutical, and veterinary drug. The 7-isomer has the highest acute toxic ity, but the other isomers are not without activity. It is gen erally advantageous to purify the 7-isomer from the less active isomers. The γ-isomer acts on the nervous system of insects, principally at the level of the nerve ganglia. As a result, lindane has been used against insects in a wide range of applications including treatment of animals, buildings, humans for ectoparasites, clothes; water for mosquitoes; living plants; seeds and soils. Some applications have been abandoned due to excessive residues, e.g., stored food stuffs. By voluntary action, the principal domestic producer of technical grade BHC requested cancellation of its BHC registrations on September 1, 1976. As of July 21, 1978, all registrants of pesticide products containing BHC voluntar ily canceled their registrations or switched their former BHC products to lindane formulations.Экологическая судьба
Soil. No biodegradation of β-BHC was observed under denitrifying and sulfate-reduc ing conditions in a contaminated soil collected from The Netherlands (Bachmann et al., 1988). In four successive 7-day incubation periods, β-BHC (5 and 10 mg/L) was recalci trant to degradation in a settled domestic wastewater inoculum (Tabak et al., 1981).Chemical/Physical. Emits very toxic fumes of chlorides, hydrochloric acid and phos gene when heated to decomposition (Lewis, 1990). β-BHC will not hydrolyze to any reasonable extent (Kollig, 1993).
Перевозки
UN2761 Organochlorine pesticides, solid, toxic, Hazard Class: 6.1; Labels: 6.1-Poisonous materials.Несовместимости
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explo sions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. Decomposes on contact with powdered iron, aluminum, zinc, and on contact with strong bases producing trichlorobenzene.Утилизация отходов
A process has been developed for the destructive pyrolysis of benzene hexachloride @ 400 500℃ with a catalyst mixture which contains 5 10% of either cupric chloride, ferric chloride; zinc chloride; or aluminum chloride on activated carbon.БЕТА-HCH запасные части и сырье
запасной предмет
БЕТА-HCH поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| 86-13657291602 | CHINA | 22963 | 58 | |
| +86-89586680 +86-13289823923 |
China | 8670 | 58 | |
| +86-852-30606658 | China | 43340 | 58 | |
| +1-+1(833)-552-7181 | United States | 57505 | 58 | |
| +86-020-81960175-617 +8615602392859 |
China | 6335 | 58 | |
| +8618950047208 | China | 43416 | 58 | |
| 400-1166-196 18981987031 |
China | 11707 | 57 | |
| 021-021-021-67601398-809-809-809 15221380277 |
China | 9658 | 60 | |
| 4008-755-333 18080918076 |
China | 9718 | 55 | |
| 4009209199 | China | 22514 | 55 |