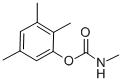
2,3,5-TRIMETHACARB
- русский язык имя
- английское имя2,3,5-TRIMETHACARB
- CAS №12407-86-2
- CBNumberCB3425785
- ФормулаC11H15NO2
- мольный вес193.24
- номер MDLMFCD00155446
- файл Mol12407-86-2.mol
химическое свойство
| Температура плавления | 118-123°C |
| Температура кипения | 329.46°C (rough estimate) |
| плотность | 1.0945 (rough estimate) |
| давление пара | 6.8 x 10-3 Pa (25 °C) |
| показатель преломления | 1.5080 (estimate) |
| температура хранения | Refrigerator |
| растворимость | Chloroform (Slightly), Methanol (Slightly) |
| Растворимость в воде | >58 mg l-1 (23 °C) |
| форма | Solid |
| цвет | White to Off-White |
| FDA UNII | R97FQQ0N7W |
| Система регистрации веществ EPA | Trimethacarb (12407-86-2) |
| РИДАДР | 2757 |
| Класс опасности | 6.1(b) |
| Группа упаковки | III |
| Токсичность | rabbit,LD50,skin,> 2500mg/kg (2500mg/kg),Shell Chemical Company. Vol. CODE, |
2,3,5-TRIMETHACARB химические свойства, назначение, производство
Использование
Trimethacarb is an insecticide with primarily stomach action. It is mainly used to control corn root worm larvae in maize but it also controls a wide range of insect and mollusc pests. Trimethacarb acts as a bird and mammal repellent.Метаболический путь
The metabolic pathways of trimethacarb in plants, mammals and insects involve mainly oxidation of N-methyl or ring-methyl substituents and conjugation. Some hydroxylation of the phenyl ring and ester hydrolysis occurs. The metabolism of trimethacarb was reviewed by Fukuto (1972), Kuhr and Dorough (1976) and by Schlagbauer and Schlagbauer (1972).Деградация
Trimethacarb is hydrolysed by strong acids and alkalis but aqueous solutions are stable to light (PM). Trimethacarb was applied to bean leaves and exposed to natural sunlight for 4 days. Both isomers were decomposed more rapidly when exposed to sunlight compared with normal light in the laboratory. The compounds were almost completely decomposed. Major photoproducts resulted from hydroxylation of methyl substituents of the phenyl ring (2 and 3) and of the N-methyl group (5 and 6) (see Schemes 1 and 2). There were few products of hydrolysis (Slade and Casida, 1970; Kuhr and Dorough, 1976).2,3,5-TRIMETHACARB поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| 18871490254 | CHINA | 28172 | 58 | |
| +86-023-6139-8061 +86-86-13650506873 |
China | 39894 | 58 | |
| United States | 24072 | 58 | ||
| +86-0512-83500002 +8615195660023 |
China | 23046 | 58 | |
| 022-65378550-8551 | China | 2773 | 58 | |
| 022-6537-8550 15522853686 |
China | 4511 | 55 | |
| 1-516-6625404 | United States | 9171 | 60 | |
| 13003551299 13003551299 |
China | 7566 | 58 |