
Формогидроксимовая кислота
- английское имяFormohydroximic acid
- CAS №4312-87-2
- CBNumberCB31247838
- ФормулаCH3NO2
- мольный вес61.04
- файл Mol4312-87-2.mol
химическое свойство
| Температура плавления | 81.5-82.0 °C |
| Температура кипения | 97.79°C (rough estimate) |
| плотность | 1.253±0.06 g/cm3(Predicted) |
| показатель преломления | 1.4400 (estimate) |
| пка | 9.13±0.23(Predicted) |
Формогидроксимовая кислота химические свойства, назначение, производство
Подготовка
(a) Preparation of hydroxylamine solution. Thirty grams (0.43 mole) of hydroxylamine hydrochloride is dissolved in 150 ml of boiling methanol. The solution is cooled somewhat and a solution of 9.9 gm (0.43 gm-atom) of sodium in 150 ml of methanol is added cautiously. The mixture is cooled and then placed in an ice-salt bath until the sodium chloride formed has been precipitated as completely as possible. After the sodium chloride has been filtered off, approximately 200 ml of the methanol is distilled off under reduced pressure. The still residue is again filtered and then used directly in the next step.(b) Preparation of formohydroxamic acid. The hydroxylamine solution, prepared as above, is cooled at 0°C. Then 31 gm (0.42 mole) of ethyl formate is added. During this addition an increase in temperature is observed. This is moderated by cooling in a stream of running water. After the addition has been completed, the reaction mixture is allowed to stand at room temperature for several hours.
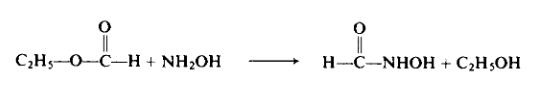
The excess methanol is evaporated from the reaction mixture under reduced pressure. The crystalline residue is contaminated with a small amount of an oily substance and some sodium chloride. The oily material is removed by pressing the crude product on an unglazed clay plate. The yield is 17.9 gm (70%). The product is crystallized from ethyl acetate. During this process, the remaining sodium chloride is separated by filtration of the hot solution. The final product melts at 81-82°C.