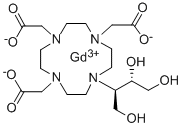
Gadobutrol
- русский язык имя
- английское имяGadobutrol
- CAS №138071-82-6
- CBNumberCB31118291
- ФормулаC18H31N4O9.Gd
- мольный вес604.712
- EINECS1308068-626-2
- номер MDLMFCD00911649
- файл Mol138071-82-6.mol
химическое свойство
| Температура плавления | >264°C (dec.) |
| плотность | 1.3 g/mL at 37 °C |
| температура хранения | Refrigerator |
| растворимость | Water (Slightly) |
| форма | Solid |
| цвет | White to Off-White |
| РН | 6.6-8 |
| ИнЧИКей | ZPDFIIGFYAHNSK-IWEVFNEWNA-K |
| SMILES | N1(CCN(CC([O-])=O)CCN(CC([O-])=O)CCN(CC([O-])=O)CC1)[C@H](CO)[C@H](O)CO.[Gd+3] |&1:24,27,r| |
| FDA UNII | 1BJ477IO2L |
| Словарь наркотиков NCI | gadobutrol |
| Код УВД | V08CA09 |
Gadobutrol химические свойства, назначение, производство
Имя бренда
Gadobutrol is marketed by Bayer AG as Gadovist, and by Bayer HealthCare Pharmaceuticals as Gadavist. In India, it is also marketed by Vivere Imaging as Viv-butrol.Побочные эффекты
Side effects of Gadobutrol are uncommon but may include: headache, nausea, vomiting, feeling unwell (malaise), dizziness, abnormal or unpleasant taste in your mouth, feeling hot, numbness or tingly feeling, itching or rash, skin redness, or injection site reactions (cold feeling, warmth, pain, or burning). Its more serious side effects include: urinating less than usual or not at all; drowsiness, confusion, mood changes, increased thirst, loss of appetite; swelling, weight gain, shortness of breath; seizures (convulsions); breathing problems; pounding heartbeats or fluttering in your chest; or severe pain, burning, or irritation around the IV needle.Утилизация отходов
Expired or waste pharmaceuticals shall carefully take into consideration applicable DEA, EPA, and FDA regulations. It is not appropriate to dispose by flushing the pharmaceutical down the toilet or discarding to trash. If possible return the pharmaceutical to the manufacturer for proper disposal being careful to properly label and securely package the material. Alternatively, the waste pharmaceutical shall be labeled, securely packaged and transported by a state licensed medical waste contractor to dispose by burial in a licensed hazardous or toxic waste landfill or incinerator.Gadobutrol поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +86-0571-85134551 | China | 15395 | 58 | ||
| +86-0571-89385087 +8613616530509 |
China | 118 | 58 | ||
| +86-0543-2240078 +8618364991597 |
China | 994 | 58 | ||
| +8618381337976 | China | 86 | 58 | ||
| 16314854226; +16314854226 |
United States | 19743 | 58 | ||
| +86-371-86557731 +86-13613820652 |
China | 20314 | 58 | ||
| +86-0371-55170693 +86-19937530512 |
China | 21670 | 55 | ||
| +undefined-21-51877795 | China | 32760 | 60 | ||
| +86-0371-86658258 +8613203830695 |
China | 29897 | 58 | ||
| +86-023-6139-8061 +86-86-13650506873 |
China | 39916 | 58 |