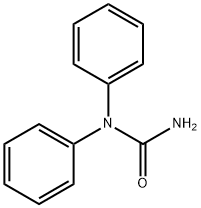
1,1-дифенилмочевина
- английское имя1,1-diphenylurea
- CAS №603-54-3
- CBNumberCB2927378
- ФормулаC13H12N2O
- мольный вес212.25
- EINECS210-048-6
- номер MDLMFCD00025460
- файл Mol603-54-3.mol
химическое свойство
| Температура плавления | 189°C |
| Температура кипения | 352.09°C (rough estimate) |
| плотность | 1.2760 |
| показатель преломления | 1.6920 (estimate) |
| пка | 15.11±0.50(Predicted) |
| Стабильность | Stable. Combustible. Incompatible with strong oxidizing agents. |
| FDA UNII | H33D27I551 |
| Система регистрации веществ EPA | 1,1-Diphenylurea (603-54-3) |
1,1-дифенилмочевина химические свойства, назначение, производство
Химические свойства
white crystalsОбщее описание
Needles or white crystals.Реакции воздуха и воды
1,1-diphenylurea may be sensitive to prolonged exposure to air or light. . Insoluble in water.Профиль реактивности
1,1-diphenylurea is an amide. Amides/imides react with azo and diazo compounds to generate toxic gases. Flammable gases are formed by the reaction of organic amides/imides with strong reducing agents. Amides are very weak bases (weaker than water). Imides are less basic yet and in fact react with strong bases to form salts. That is, they can react as acids. Mixing amides with dehydrating agents such as P2O5 or SOCl2 generates the corresponding nitrile. The combustion of these compounds generates mixed oxides of nitrogen (NOx).Пожароопасность
Flash point data for 1,1-diphenylurea are not available, but 1,1-diphenylurea is probably combustible.Методы очистки
Crystallise 1,1-diphenylurea from MeOH. [Beilstein for 1,3 12 IV 741.]