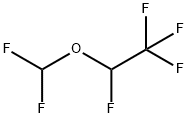
Десфлуран
- английское имяDesflurane
- CAS №57041-67-5
- CBNumberCB2304120
- ФормулаC3H2F6O
- мольный вес168.04
- номер MDLMFCD00236716
- файл Mol57041-67-5.mol
химическое свойство
| Температура кипения | 23-24°C |
| плотность | 1,47 g/cm3 |
| показатель преломления | 1.3577 (estimate) |
| растворимость | Practically insoluble in water, miscible with anhydrous ethanol. |
| форма | liquid |
| цвет | Clear |
| Справочник по базе данных CAS | 57041-67-5(CAS DataBase Reference) |
| FDA UNII | CRS35BZ94Q |
| Словарь наркотиков NCI | desflurane |
| Код УВД | N01AB07 |
| Система регистрации веществ EPA | Ethane, 2-(difluoromethoxy)-1,1,1,2-tetrafluoro-, (+-)- (57041-67-5) |
| UNSPSC Code | 41116107 |
| NACRES | NA.24 |
| Коды опасности | Xi | |||||||||
| Заявления о безопасности | 23 | |||||||||
| Примечание об опасности | Irritant | |||||||||
| Класс опасности | GAS | |||||||||
| кода HS | 2909191800 | |||||||||
| Банк данных об опасных веществах | 57041-67-5(Hazardous Substances Data) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H315:При попадании на кожу вызывает раздражение.
H319:При попадании в глаза вызывает выраженное раздражение.
H335:Может вызывать раздражение верхних дыхательных путей.
-
оператор предупредительных мер
P261:Избегать вдыхания пыли/ дыма/ газа/ тумана/ паров/ аэрозолей.
P271:Использовать только на открытом воздухе или в хорошо вентилируемом помещении.
P280:Использовать перчатки/ средства защиты глаз/ лица.
Десфлуран химические свойства, назначение, производство
Описание
Desflurane is a new inhalation anesthetic introduced for induction and maintenance of general anesthesia in adults. Due to reports of respiratory irritation, desflurane may be used only for maintenance in children. Although almost structurally identical to isoflurane, a halogen replacement gives desflurane an improved pharmacokinetic profile. It is less soluble in blood and tissue and produces a fast onset of action and a more rapid recovery from anesthesia.Химические свойства
Desflurane is a clear, colourless, mobile, heavy liquid. It is similar to isoflurane (CHF2-O-CHCl-CF3) in that both are halogenated compounds of methyl ethane, except that chlorine is replaced with fluorine in the α-ethyl portion. The halogenation of fluorine reduces the solubility of blood and tissues and alters the boiling point, vapor pressure and stability of desflurane, enhancing its molecular stability as well as its resistance to biodegradation and alkaline degradation.История
Desflurane was first synthesized by Russel et al U.S. at the 29. of July 1975. The synthesis was started by using floural methyl hemiacetal (CF3CH(OH)OCH3) and converting it to 1,2,2,2- tetraflouroethyl methyl ether (CF3CHFOCH3). Next step was chlorinating two hydrogen atoms of the methyl-group to CF3CHFOCHCl2. This next to last molecule was charged with HF in the presence of antimony pentachloride (SBCL5) to Desflurane. But this way of synthesizing Desfluran was not usable for industrial synthesis, because of the less yield of Desflurane and the expensive educts.Использование
Anesthetic.Подготовка
Isoflurane and bromine trifluoride were reacted overnight at room temperature to synthesize desflurane in 62% yield.Биологические функции
Desflurane (Suprane) shares most of the pharmacological properties of isoflurane. Desflurane has low tissue and blood solubility compared with other halogenated hydrocarbons, and its anesthetic partial pressure is thus established more rapidly. Recovery is similarly prompt when the patient is switched to room air or oxygen. Desflurane’s popularity for outpatient procedures stems from its rapid onset and prompt elimination from the body by exhalation. A disadvantage is that desflurane irritates the respiratory tract; thus, it is not preferred for induction of anesthesia using an inhalational technique. However, desflurane may be used to maintain anesthesia after induction with an alternative IV or inhalational agent, preserving the advantage of rapid recovery.Desflurane, like other halogenated hydrocarbon anesthetics, causes a decrease in blood pressure.The reduced pressure occurs primarily as a consequence of decreased vascular resistance, and since cardiac output is well maintained, tissue perfusion is preserved.
Desflurane stimulates the sympathetic nervous system and causes abrupt transient tachycardia during induction or as the concentration of the agent is raised to meet the patient’s changing needs.
Desflurane causes an increase in the rate of ventilation, a decrease in tidal volume, and a decrease in minute volume as inspired concentrations only slightly exceed 1 MAC. Thus should anesthesiologists require desflurane to be administered near or above MAC levels, patients are likely to have marked reductions in PCO2.
Общее описание
Desflurane is a nonflammable, colorless, very volatile liquidpackaged in amber-colored vials. The boiling point is22.8°C, and it requires a vaporizer specifically designed fordesflurane. The manufacturer states that the vials can bestored at room temperature. Desflurane has a blood:gas partitioncoefficient of 0.42, an MAC of 7.3% and an oil:gaspartition coefficient of 18.7. The low blood:gas partition coefficientleads to fast induction times and short recoverytimes. Desflurane is not recommended for induction anesthesiain children because of the high incidence of laryngospasms(50%), coughing (72%), breath holding (68%),and increase in secretions (21%). Desflurane can producea dose-dependent decrease in blood pressure and concentrationsexceeding 1 MAC may cause transient increases inheart rate. Desflurane can react with desiccated carbon dioxideabsorbents to produce carbon monoxide that may resultin elevated levels of carboxyhemoglobin.24.Метаболизм
Desflurane is not metabolized to any great extent and, therefore, has not been associated with hepatotoxicity or nephrotoxicity. Metabolites, mostly trifluoroacetate, account for less than 0.02% of the administered dose. Whereas desflurane can react with soda lime or Baralyme to form carbon monoxide, no reports of adverse outcomes in patients have appeared.Десфлуран запасные части и сырье
Десфлуран поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86-0371-86658258 +8613203830695 |
China | 30233 | 58 | |
| +86-0551-65418671 +8618949823763 |
China | 34563 | 58 | |
| +86-29-89586680 +86-15129568250 |
China | 22787 | 58 | |
| +86-0571-85134551 | China | 15352 | 58 | |
| +86-0512-83500002 +8615195660023 |
China | 23046 | 58 | |
| 571-89925085 | China | 131957 | 58 | |
| +86-0592-6210733 | China | 32343 | 55 | |
| 010-82848833 400-666-7788 |
China | 94657 | 76 | |
| 1-(800)-881-8210 | United States | 2123 | 70 | |
| +91-22-26355700 | India | 9679 | 58 |