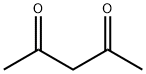
Ацетилацетон
- английское имяAcetylacetone
- CAS №123-54-6
- CBNumberCB2179401
- ФормулаC5H8O2
- мольный вес100.12
- EINECS204-634-0
- номер MDLMFCD00008787
- файл Mol123-54-6.mol
| Температура плавления | -23 °C (lit.) |
| Температура кипения | 140.4 °C (lit.) |
| плотность | 0.975 g/mL at 25 °C (lit.) |
| плотность пара | 3.5 (vs air) |
| давление пара | 6 mm Hg ( 20 °C) |
| показатель преломления | n |
| Fp | 66 °F |
| температура хранения | Store below +30°C. |
| растворимость | H2O: soluble1 in 8 parts |
| пка | 8.9(at 25℃) |
| форма | Liquid |
| цвет | very deep green-yellow |
| Относительная полярность | 0.571 |
| РН | 6 (200g/l, H2O, 20℃) |
| Запах | pleasant odor |
| Пределы взрываемости | 2.4-11.4%(V) |
| Скорость испарения | 0.75 |
| Относительная плотность газов(воздух=1) | 3.5 |
| Растворимость в воде | 16 g/100 mL (20 ºC) |
| Мерк | 14,81 |
| БРН | 741937 |
| Пределы воздействия | No exposure limit has been set. |
| Диэлектрическая постоянная | 23.1(20℃) |
| ИнЧИКей | YRKCREAYFQTBPV-UHFFFAOYSA-N |
| LogP | 0.68 at 20℃ |
| Непрямые добавки, используемые в веществах, контактирующих с пищевыми продуктами | 2,4-PENTANEDIONE |
| FDA 21 CFR | 175.105 |
| Справочник по базе данных CAS | 123-54-6(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 2 |
| FDA UNII | 46R950BP4J |
| Справочник по химии NIST | Acetylacetone(123-54-6) |
| Система регистрации веществ EPA | Acetylacetone (123-54-6) |
| UNSPSC Code | 41116107 |
| NACRES | NA.24 |
| Коды опасности | Xn,Xi | |||||||||
| Заявления о рисках | 11-36/37/38-22-10-R22-R10-20/21/22-2017/10/22 | |||||||||
| Заявления о безопасности | 21-23-24/25-36-26-S24/25-S23-S21 | |||||||||
| РИДАДР | UN 2310 3/PG 3 | |||||||||
| WGK Германия | 1 | |||||||||
| RTECS | SA1925000 | |||||||||
| F | 9-23 | |||||||||
| Температура самовоспламенения | 662 °F | |||||||||
| TSCA | Yes | |||||||||
| кода HS | 2914 19 90 | |||||||||
| Класс опасности | 3 | |||||||||
| Группа упаковки | III | |||||||||
| Банк данных об опасных веществах | 123-54-6(Hazardous Substances Data) | |||||||||
| Токсичность | LC50 (4 hrs) in rats: 1000 ppm (Carpenter) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
опасность
-
вредная бумага
H302:Вредно при проглатывании.
H226:Воспламеняющаяся жидкость. Пары образуют с воздухом взрывоопасные смеси.
H311+H331:Токсично при попадании на кожу или при вдыхании.
-
оператор предупредительных мер
P210:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P301+P312+P330:ПРИ ПРОГЛАТЫВАНИИ: Обратиться за медицинской помощью при плохом самочувствии. Прополоскать рот.
P302+P352+P312:ПРИ ПОПАДАНИИ НА КОЖУ: Промыть большим количеством воды. Обратиться за медицинской помощью при плохом самочувствии.
P304+P340+P311:ПРИ ВДЫХАНИИ: Свежий воздух, покой. Обратиться за медицинской помощью.
Ацетилацетон химические свойства, назначение, производство
Описание
Acetylacetone (2,4-pentanedione) is a clear or slightly yellowish liquid with a putrid odour. It is readily soluble in water and in organic solvents and incompatible with light, ignition sources, excess heat, oxidising agents, strong reducing agents, and strong bases. On decomposition, acetylacetone releases hazardous products such as carbon monoxide, irritating and toxic fumes and gases, and carbon dioxide. Acetylacetone is used in the production of anti-corrosion agents and its peroxide compounds for the radical initiator application for polymerisation. It is used as a chemical intermediate for drugs (such as sulphamethazine, nicarbazine, vitamin B6, and vitamin K) and pesticides sulfonylurea herbicides and pesticides. It is used as an indicator for the complexometric titration of Fe (III), for the modification of guanidino groups and amino groups in proteins, and for the preparation of metal acetylacetonates for catalyst application.Химические свойства
Acetylacetone (2,4-pentanedione) is a clear or slightly yellowish liquid with a putrid odor. It is readily soluble in water. It is with other incompatible materials, light, ignition sources, excess heat, oxidizing agents, strong reducing agents, and strong bases. On decomposition, acetylacetone releases hazardous products, such as carbon monoxide, irritating and toxic fumes and gases, and carbon dioxide. Acetylacetone is used in the production of anticorrosion agents and its peroxide compounds for the radical initiator application for polymerization. It is used as a chemical intermediate for drugs (such as sulfamethazine, nicarbazine, vitamin B6, and vitamin K), sulfonylurea herbicides, and pesticides. It is used as a solvent for cellulose acetate, as an additive in gasoline and lubricant, as a dryer of paint and varnish. It is used as an indicator for the complexometric titration of Fe(III), for the modifi cation of guanidino groups and amino groups in proteins, and in the preparation of metal acetylacetonates for catalyst application.Химические свойства
Acetylacetone is a colorless to yellowish liquid with a sour, rancid odor. The Odor Threshold is 0.01 ppm. Or Ethereal-minty odor, somewhat metallic or "chemical". In high dilution, the flavor in aqueous medium is sweet, remotely reminiscent of Peppermint sweetness. Not very stable. The enol form readily passes into equilibrium mixture. Prod. from Acetone plus Ethyl acetate, or Acetone plus Acetic anhydride (with Boron trifluoride).Использование
Acetylacetone was used in preparing Y203, La203 and La2CuO4 thin films and the titanate/anatase dual-phase photocatalyst.Определение
ChEBI: A beta-diketone that is pentane in which the hydrogens at positions 2 and 4 are replaced by oxo groups.прикладной
Acetylacetone, also known as 2,4-pentanedione, is an important commodity chemical and widely used as a fuel additive, as dyeing intermediate, in the fields of metal extraction, metal plating, and resin modification. Hantzsch reaction was used as a derivatizing agent for the assay of compounds having a primary amino group. The reagent was reacted with the primary amino group of the drugs to form a product having color and/or emit fluorescence. This condensation reaction was distinguished by its precision, reproducibility, and analytical cost reduction. FLX contains an aliphatic amino group, in the presence of formaldehyde solution, this amino group can condense with two equivalents of acetylacetone to form dihydropyridine derivative that emits yellow fluorescent product. (Figure1). Under optimized conditions of the reaction, FLX gave highly fluorescent product measured at λem 479 nm using 419 nm as excitation.Методы производства
2,4-Pentanedione is produced by thermal or metal-catalyzed rearrangement of isopropenyl acetate(obtained from acetone and ketene):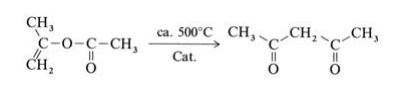
Isopropenyl acetate vapor is fed at atmospheric pressure through a V2A steel tube with an inner temperature of 520℃. The hot reaction gases are quenched, condensed, and cooled to 20℃, whereby the gaseous byproducts carbon monoxide, carbon dioxide, methane, and ketene are separated. The product is purified by fractional distillation. Other industrially less important processes for the production of 2,4-pentanedione, include the Claisen ester condensation of ethyl acetate with acetone using sodium ethoxide as condensation agent and the acetylation of acetoacetic acid esters with acetic anhydride in the presence of magnesium salts.
Общее описание
A colorless or yellow colored liquid. Less dense than water. Flash point 105°F. Vapors are heavier than air. Used as a solvent in paints and varnishes.Реакции воздуха и воды
Flammable. Soluble in water.Профиль реактивности
Ketones, such as 2,4-Pentanedione, are reactive with many acids and bases liberating heat and flammable gases (e.g., H2). The amount of heat may be sufficient to start a fire in the unreacted portion of the ketone. Ketones react with reducing agents such as hydrides, alkali metals, and nitrides to produce flammable gas (H2) and heat. Ketones are incompatible with isocyanates, aldehydes, cyanides, peroxides, and anhydrides. They react violently with aldehydes, HNO3, HNO3 + H2O2, and HClO4. May dissolve plastics [USCG, 1999].Пожароопасность
Behavior in Fire: Vapor is heavier than air and may travel to a source of ignition and flash back.Профиль безопасности
Poison by ingestion and intraperitoneal routes. Moderately toxic by inhalation. A skin and severe eye irritant. Experimental reproductive effects. Mutation data reported. Flammable liquid when exposed to heat or flame. Incompatible with oxidning materials. To fight fire, use alcohol foam, CO2, dry chemical.Возможный контакт
Acetoacetic acid derivative. 2,4-Pentanedione is used in gasoline and lubricant additives, fungicides, insecticides, and colors manufacture; as a chemical intermediate and in the manufacture of metal chelatesхранилище
Acetylacetone should be stored away from heat, sparks, flame, and from sources of ignition. It should be stored in a tightly sealed container, in a cool, dry, well-ventilated area, away from incompatible substances.Перевозки
UN2310 Pentane-2,4-dione, Hazard Class: 3; Labels: 3-Flammable liquidМетоды очистки
Small amounts of acetic acid are removed by shaking with small portions of 2M NaOH until the aqueous phase remains faintly alkaline. The sample, after washing with water, is dried with anhydrous Na2SO4, and distilled through a modified Vigreux column (p 11) Cartledge J Am Chem Soc 73 4416 1951]. An additional purification step is fractional crystallisation from the liquid. Alternatively, there is less loss of acetylacetone if it is dissolved in four volumes of *benzene and the solution is shaken three times with an equal volume of distilled water (to extract acetic acid): the *benzene is then removed by distillation at 43-53o and 20-30mm through a helices-packed column. It is then refluxed over P2O5 (10g/L) and fractionally distilled under reduced pressure. The distillate (sp conductivity 4 x 10-8 ohm-1cm-1) is suitable for polarography [Fujinaga & Lee Talanta 24 395 1977]. To recover used acetylacetone, metal ions are stripped from the solution at pH 1 (using 100mL 0.1M H2SO4/L of acetylacetone). The acetylacetone is then washed with (1:10) ammonia solution (100mL/L) and with distilled water (100mL/L, twice), then treated as above. It complexes with Al, Be, Ca, Cd, Ce , Cu, Fe2+, Fe3+ , Mn, Mg, Ni, Pb and Zn. [Beilstein 1 H 777, 1 I 401, 1 II 831, 1 III 3113, 1 IV 3662.]Несовместимости
Vapors may form explosive mixture with air. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. reducing agents; halogens, aliphatic amines; alkanolamines, organic acids; isocyanates. Strong light may cause polymerization.Утилизация отходов
Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed.Меры предосторожности
Occupational workers should only use/handle acetyl acetone in a well-ventilated area, with spark-proof tools and explosion-proof equipment. Workers should not cut, weld, braze, solder, drill, grind, pressurize, or expose empty containers to heat, sparks, or flames.Ацетилацетон запасные части и сырье
сырьё
1of3
запасной предмет
- Tetrahydrocurcumin
- 5-ЦИАНО-6-ГИДРОКСИ-4-МЕТОКСИМЕТИЛ-2-МЕТИЛПИРИДИН
- Алюминия ацетилацетона
- 17-Ethinyl-3,17-dihydroxy-18-methylestra-2,5(10)-diene3-methylether
- Нингидрин
- 3-(3,5-ДИМЕТИЛ-ПИРАЗОЛ-1-ИЛ)-ПРОПИЛАМИН
- 1,2-DIHYDRO-4-(METHOXYMETHYL)-6-METHYL-5-NITRO-2-OXONICOTINONITRILE
- 4-methoxymethylpyridoxine
- 2-АМИНО-4,6-ДИМЕТИЛ-3-ПИРИДИНКАРБОКСАМИД
- 5-БРОМ-2-ХЛОР-4,6-ДИМЕТИЛНИКОТИНОНИТРИЛ
- 4,6-Dimethyl-2-methylmercapyrimidine
- 4,6-диметил-пиримидин-2-сульфонил фторид
- 1,4,6-Trimethyl-1H-pyrazolo[3,4-b]pyridin-3-ylamine ,97%
- 2-(2-КАРБОКСИЭТИЛ)ТИО-4,6-ДИМЕТИЛПИРИМИДИН
- 4,6-DIMETHYL-2-THIOPYRIMIDINE
- 2- (КАРБОКСИМЕТИЛТИО) -4,6-ДИМЕТИЛПИРИМИДИН МОНОГИДРАТ
- 13-Ethyl-17-hydroxy-18,19-dinorpregn-5(10)-en-20-yn-3-one
- 5-ацетил-2 ,4-диметилтиазол
- Палладий(II) ацетилацетонат
- 4,6-DIMETHYL-2-HYDROXYPYRIMIDINE HYDROCHLORIDE
- 3-Циано-2-гидрокси-4 ,6-диметилпиридин
- 2-Этил-3-метилпиразина
- MEQUINDOX
- 6-CHLORO-5-CYANO-4-METHOXYMETHYL-3-NITRO-2-PICOLINE
- 17-Ethinyl-17-hydroxy-18-methylestra-5(10),9(11)-dien-3-one-3-ethylene ketal
- 2-амино-4 ,6-диметилпиримидина
- 1-(4-METHYL-2-(METHYLTHIO)PYRIMIDIN-5-YL)ETHANONE
- 3-(PYRIMIDIN-2-YLTHIO)PENTANE-2,4-DIONE
- 1-(2,4-DIMETHYLQUINOLIN-3-YL)ETHANONE HYDROCHLORIDE
- 2-Хлор-3-циано-4 ,6-диметилпиридин
1of8
Ацетилацетон поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| 86-22-66880623 +8618622897568 |
China | 571 | 58 | ||
| +8613367258412 | China | 10319 | 58 | ||
| +86-0519-8359-8696 +8618018249389 |
China | 9897 | 58 | ||
| +8615531157085 | China | 8804 | 58 | ||
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | ||
| +86-13131129325 | China | 5887 | 58 | ||
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | ||
| +86-021-57951555 +8617317452075 |
China | 1803 | 55 | ||
| +86-25-86655873 +8613962173137 |
China | 193 | 55 | ||
| +86-0551-65418679 +8618949832763 |
China | 2986 | 55 |