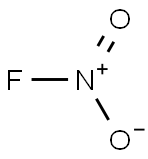
nitryl fluoride
- русский язык имя
- английское имяnitryl fluoride
- CAS №10022-50-1
- CBNumberCB1906505
- ФормулаFNO2
- мольный вес65
- EINECS233-021-0
- файл Mol10022-50-1.mol
химическое свойство
| Температура плавления | -166.0° |
| Температура кипения | bp -72.4° |
| плотность | (liq at bp) 1.796; d (solid) 1.924 |
| растворимость | reacts with H2O |
| форма | colorless gas |
| цвет | Colorless gas |
| Запах | pungent odor |
| Растворимость в воде | hydrolyzes with HNO3 and HF as products [HAW93] |
| Справочник по базе данных CAS | 10022-50-1 |
| FDA UNII | DAT2I9R64A |
nitryl fluoride химические свойства, назначение, производство
Химические свойства
Colorless gas.Strong oxidizing agent; hydrolyzes to form nitric and hydrogen fluoride acids.Физические свойства
Colorless gas; pungent odor; density 2.90 g/L; heavier than air, density in air 2.24 (air=1); liquefies to a colorless liquid at -63.5°C; solidifies at -139°C; decomposes in water; also decomposes in alcohol, ether and chloroform.Использование
Oxidizer in rocket propellants; fluorinating agent.Опасность
Ignites on contact with selenium, iodine, phosphorus, arsenic, antimony, boron, silicon, molybdenum. Corrosive to tissue.Профиль безопасности
Poison by inhalation. A severe irritant to skin, eyes, and mucous membranes. A powerful oxidlzing agent. This gas is intensely reactive. Explosive reaction with hydrogen at 200-300°C. Ignites on contact with antimony, arsenic, boron, iodme, phosphorus, selenium. Ignites when warmed with bismuth, carbon, chromium, lead, sulfur. Incandescent reaction with aluminum, cadmium, cobalt, iron, molybdenum, nickel, potassium, sodium, thorium, titanium, tungsten, uran uranium, vanadlum, zinc, zirconium, lithium (at 200-300°C), manganese (at 200-300°C). Incompatible with metals, nonmetals. When heated to decomposition it emits toxic fumes of Fand NOx. See also FLUORIDES.nitryl fluoride запасные части и сырье
запасной предмет