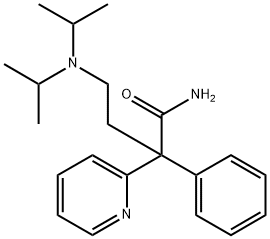
DISOPYRAMIDE
- русский язык имя
- английское имяDISOPYRAMIDE
- CAS №3737-09-5
- CBNumberCB1285468
- ФормулаC21H29N3O
- мольный вес339.48
- EINECS223-110-2
- номер MDLMFCD00057366
- файл Mol3737-09-5.mol
химическое свойство
| Температура плавления | 94.5-950C |
| Температура кипения | 475.43°C (rough estimate) |
| плотность | 1.0779 (rough estimate) |
| показатель преломления | 1.6300 (estimate) |
| температура хранения | Inert atmosphere,Room Temperature |
| растворимость | Soluble in DMSO (25 mg/ml) and Ethanol (>35 mg/mL) |
| форма | solid |
| пка | 10.2; also reported as 10.45(at 25℃) |
| цвет | White |
| Растворимость в воде | 6.17mg/L(22.5 ºC) |
| Мерк | 14,3360 |
| Стабильность | Stable for 1 year from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20°C for up to 1 month. |
| Справочник по базе данных CAS | 3737-09-5(CAS DataBase Reference) |
| FDA UNII | GFO928U8MQ |
| Код УВД | C01BA03 |
| UNSPSC Code | 41116107 |
| NACRES | NA.24 |
| Коды опасности | Xn,Xi,T,F | |||||||||
| Заявления о рисках | 22-36/37/38-39/23/24/25-23/24/25-11 | |||||||||
| Заявления о безопасности | 36-26-45-36/37-16-7 | |||||||||
| WGK Германия | 3 | |||||||||
| RTECS | UR8432000 | |||||||||
| кода HS | 2933.39.4100 | |||||||||
| Токсичность | LD50 i.p. in mice: 517 mmol/kg (Ruenitz, Mokler) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
предупреждение
-
вредная бумага
H302:Вредно при проглатывании.
H361:Предполагается, что данное вещество может отрицательно повлиять на способность к деторождению или на неродившегося ребенка.
-
оператор предупредительных мер
P201:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P301+P312+P330:ПРИ ПРОГЛАТЫВАНИИ: Обратиться за медицинской помощью при плохом самочувствии. Прополоскать рот.
P308+P313:ПРИ подозрении на возможность воздействия обратиться за медицинской помощью.
DISOPYRAMIDE химические свойства, назначение, производство
Описание
Structurally, disopyramide does not belong to any of the known classes of antiarrhythmics; however, being a drug of the class IA sodium channel blockers, it exhibits membranestabilizing action and increases the effective refractory period and duration of an action potential in the atrium and ventricles. It causes a decrease in contractability and excitability of the myocardium, slowing of conductivity, and suppression of sinoatride automatism.Химические свойства
Crystalline SolidИспользование
Disopyramide is used for preventing and restoring atrial and ventricular extrasystole and tachycardia in order to prevent atrial flutter and arrhythmia.Определение
ChEBI: A monocarboxylic acid amide that is butanamide substituted by a diisopropylamino group at position 4, a phenyl group at position 2 and a pyridin-2-yl group at position 2. It is used as a anti-arrhythmia drug.Фармакокине?тика
Disopyramide phosphate is used orally for the treatment of certain ventricular and atrial arrhythmias. Despite its structural dissimilarity to procainamide, its cardiac effects are very similar. Disopyramide is rapidly and completely absorbed from the gastrointestinal tract. Peak plasma level is usually reached within 1 to 3 hours, and a plasma half-life of 5 to 7 hours is common. Approximately half of an oral dose is excreted unchanged in the urine. The remaining drug undergoes hepatic metabolism, principally to the corresponding N-dealkylated form. This metabolite retains approximately half the antiarrhythmic activity of disopyramide and also is subject to renal excretion.Клиническое использование
Disopyramide (Norpace) can suppress atrial and ventricular arrhythmias and is longer acting than other drugs in its class.The indications for use of disopyramide are similar to those for quinidine, except that it is not approved for use in the prophylaxis of atrial flutter or atrial fibrillation after DC conversion.The indications are as follows: unifocal premature (ectopic) ventricular contractions, premature (ectopic) ventricular contractions of multifocal origin, paired premature ventricular contractions (couplets), and episodes of ventricular tachycardia. Persistent ventricular tachycardia is usually treated with DC conversion.
Побочные эффекты
The major toxic reactions to disopyramide administration include hypotension, congestive heart failure, and conduction disturbances. These effects are the result of disopyramide’s ability to depress myocardial contractility and myocardial conduction. Although disopyramide initially may produce ventricular tachyarrhythmias or ventricular fibrillation in some patients, the incidence of disopyramide-induced syncope in long-term therapy is not known. Most other toxic reactions (e.g., dry mouth, blurred vision, constipation) can be attributed to the anticholinergic properties of the drug.CNS stimulation and hallucinations are rare.The incidence of severe adverse effects in long-term therapy may be lower than those observed with quinidine or procainamide.
взаимодействия лекарств
In the presence of phenytoin, the metabolism of disopyramide is increased (reducing its effective concentration) and the accumulation of its metabolites is also increased, thereby increasing the probability of anticholinergic adverse effects. Rifampin also stimulates the hepatic metabolism of disopyramide, reducing its plasma concentration.Unlike quinidine, disopyramide does not increase the plasma concentration of digoxin in patients receiving a maintenance dose of the cardiac glycoside. Hypoglycemia has been reported with the use of disopyramide, particularly in conjunction with moderate or excessive alcohol intake.
Меры предосторожности
Disopyramide should not be administered in cardiogenic shock, preexisting second- or third-degree A-V block, or known hypersensitivity to the drug. Neither should it be given to patients who are poorly compensated or those with uncompensated heart failure or severe hypotension. Because of its ability to slow cardiac conduction, disopyramide is not indicated for the treatment of digitalis-induced ventricular arrhythmias.Patients with congenital prolongation of the QT interval should not receive quinidine, procainamide, or disopyramide because further prolongation of the QT interval may increase the incidence of ventricular fibrillation. Because of its anticholinergic properties, disopyramide should not be used in patients with glaucoma. Urinary retention and benign prostatic hypertrophy are also relative contraindications to disopyramide therapy. Patients with myasthenia gravis may have a myasthenic crisis after disopyramide administration as a result of the drug’s local anesthetic action at the neuromuscular junction.The elderly patient may exhibit increased sensitivity to the anticholinergic actions of disopyramide. Caution is advised when disopyramide is used in conjunction with other cardiac depressant drugs, such as verapamil, which may adversely affect atrioventricular conduction.
DISOPYRAMIDE запасные части и сырье
DISOPYRAMIDE поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86-029-81138252 +86-18789408387 |
China | 3889 | 58 | |
| +1-781-999-5354 +1-00000000000 |
United States | 32161 | 58 | |
| +86-0371-86658258 +8613203830695 |
China | 30233 | 58 | |
| +86-029-89586680 +86-18192503167 |
China | 7724 | 58 | |
| +8617013299288 | China | 12373 | 58 | |
| United States | 38631 | 58 | ||
| +1-+1(833)-552-7181 | United States | 57505 | 58 | |
| +8618950047208 | China | 43416 | 58 | |
| +86-0592-6210733 | China | 32343 | 55 | |
| 010-82848833 400-666-7788 |
China | 94657 | 76 |