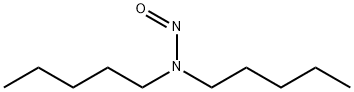
N,N-Diamylnitrosamine
- русский язык имя
- английское имяN,N-Diamylnitrosamine
- CAS №13256-06-9
- CBNumberCB11176514
- ФормулаC10H22N2O
- мольный вес186.29
- файл Mol13256-06-9.mol
химическое свойство
| Температура кипения | 320.8°C (rough estimate) |
| плотность | 0.9698 (rough estimate) |
| показатель преломления | 1.4710 (estimate) |
| температура хранения | Refrigerator |
| растворимость | Chloroform (Slightly), Ethyl Acetate (Slightly) |
| форма | Oil |
| пка | -3.15±0.70(Predicted) |
| цвет | Clear Colourless to Pale Yellow |
| Стабильность | Stable. Incompatible with strong oxidizing agents. |
| Справочник по базе данных CAS | 13256-06-9 |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | 7Q89WR197I |
| Система регистрации веществ EPA | N,N,-Diamylnitrosamine (13256-06-9) |
N,N-Diamylnitrosamine химические свойства, назначение, производство
Химические свойства
colourless to light yellow liquidИспользование
N,N-Diamylnitrosamine (cas# 13256-06-9) is a compound useful in organic synthesis.Общее описание
Clear light yellow liquid.Реакции воздуха и воды
Insoluble in water.Профиль реактивности
A nitrated amine. Amines are combustible. The combustion of amines yields noxious NOx. REACTIVITY - Amines are chemical bases. They neutralize acids to form salts plus water. These acid-base reactions are exothermic. The amount of heat that is evolved per mole of amine in a neutralization is largely independent of the strength of the amine as a base. Amines may be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen is generated by amines in combination with strong reducing agents, such as hydrides. Aromatic nitro compounds range from slight to strong oxidizing agents. If mixed with reducing agents, including hydrides, sulfides and nitrides, they may begin a vigorous reaction that culminates in a detonation. The aromatic nitro compounds may explode in the presence of a base such as sodium hydroxide or potassium hydroxide even in the presence of water or organic solvents. The explosive tendencies of aromatic nitro compounds are increased by the presence of multiple nitro groups.N,N-Diamylnitrosamine запасные части и сырье
сырьё
N,N-Diamylnitrosamine поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| 010-82848833 400-666-7788 |
China | 94657 | 76 | |
| 021-50135380 | China | 32321 | 50 | |
| 025-66023220 0086-15250997978 |
China | 7892 | 55 | |
| 025-66113011 18112977050 |
China | 15494 | 58 | |
| +86-400-002-6226 +86-13028896684; |
China | 57423 | 58 | |
| 18762407961 | China | 793 | 58 | |
| 021-58432009 400-005-6266 |
China | 44807 | 58 | |
| 18162595016; 18162595016 |
China | 10335 | 58 | |
| 18917019315 18917019315 |
China | 9332 | 58 | |
| -- | United Kingdom | 6005 | 58 |