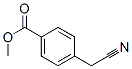
Метил-4- (цианометил) бензоат
- английское имяCyanide
- CAS №57-12-5
- CBNumberCB11075200
- ФормулаCHN
- мольный вес26.02
- файл Mol57-12-5.mol
химическое свойство
| Температура плавления | 148 °C (decomp) |
| Растворимость в воде | Miscible with water. |
| Стандарт первичной питьевой воды EPA | MCL:0.2,MCLG:0.2 |
| FDA 21 CFR | 165.110 |
| Справочник по базе данных CAS | 57-12-5 |
| Рейтинг продуктов питания EWG | 3 |
| FDA UNII | OXN4E7L11K |
| Система регистрации веществ EPA | Cyanide (57-12-5) |
| РИДАДР | 1588 |
| OEL | STEL: 4.7 ppm; 5.0 mg/m3 |
| Класс опасности | 6.1(a) |
| Группа упаковки | II |
Метил-4- (цианометил) бензоат химические свойства, назначение, производство
Химические свойства
Cyanides comprise a wide range of compounds, all of which have a CN molecule and exist in either a solid, liquid, or gaseous form. Physical characteristics range froma colorless or pale blue liquid with a faint bitter almond-like odor (hydrogen cyanide, HCN) to a white solid, powder, or crystalline hygroscopic salt (sodium cyanide, NaCN) to a colorless toxic gas also with an almond-like odor (cyanogens, NCCN). Cyanide compounds are either organic or inorganic. Organic cyanides contain a noncovalent CN functional group and are typically called the cyano group or nitriles. A common nitrile is methyl cyanide, also known as acetonitrile (CH3CN). Inorganic cyanides have a negatively charged polyatomic cyanide ion (CN) and are generally referred to as cyanides. This group includes the cyanide salts (i.e., sodium cyanide, NaCN) which are considered the most toxic form.KCN and NaCN are white crystalline solids with a faint almond odor. Sodium cyanide also has a slight odor of hydrocyanic acid when damp. KCN: boiling point=1625℃ ; freezing/melting point=634℃ . NaCN: boiling point 1496℃; freezing/melting point=564℃ . NFPA 704 M Hazard identification (KCN and NaCN): Health 3, flammability 0 , reactivity 1. Soluble in water; slow decomposition releases highly toxic and flammable hydrogen cyanide gas.
Использование
Cyanide, standard solution is used to dissolve metals and their ores. It is also used in clinical chemistry and in waste water treatment facilities to determine the concentration of dissolved and particulate materials.Методы производства
Hydrogen cyanide is generally produced at point of use. Production is covered under 40 CFR 415.420, applicability; description of the hydrogen cyanide production subcategory.Определение
ChEBI: A pseudohalide anion that is the conjugate base of hydrogen cyanide.Общее описание
Aqueous solutions with a faint odor of bitter almonds. Toxic by skin absorption, by ingestion, and inhalation of the hydrogen cyanide from the decomposition of the material. Toxic oxides of nitrogen are produced in fires involving Methyl4-(cyanomethyl)benzoate. Obtain the technical name of the material from the shipping papers and contact CHEMTREC, 800-424-9300 for specific response information.Реакции воздуха и воды
Water soluble. Inorganic cyanides react slowly with water to evolve gaseous hydrogen cyanide (HCN).Профиль реактивности
CYANIDE SOLUTIONS slowly evolve hydrogen cyanide, a flammable and poisonous gas. Acids cause the rapid evolution of HCN. Carbon dioxide from the air is sufficiently acidic to liberate HCN from solutions of cyanides. Incompatible with isocyanates, nitrides, and peroxides. Mayinitiate polymerization reactions of epoxides. May react exothermically with metal salts to produce explosive products or evolve gaseous hydrogen.Опасность
Cellular asphyxiation, respiration inhibition, highly toxic; very poisonous.Угроза здоровью
TOXIC; inhalation, ingestion or contact (skin, eyes) with vapors, dusts or substance may cause severe injury, burns or death. Reaction with water or moist air will release toxic, corrosive or flammable gases. Reaction with water may generate much heat that will increase the concentration of fumes in the air. Fire will produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.Пожароопасность
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Vapors may accumulate in confined areas (basement, tanks, hopper/tank cars etc.). Substance will react with water (some violently), releasing corrosive and/or toxic gases and runoff. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated or if contaminated with water.Профиль безопасности
Very poisonous by most routes. Cyanide directly stimulates the chemoreceptors of the carotid and aortic bodies with a resultant hyperpnea (increase in the depth and rate of respiration). Cardac irregularities are often noted, but the heart invariably outlasts the respirations. Death is due to respiratory arrest of central origm. It can occur withn seconds or minutes of the inhalation of htgh concentrations of HCN gas. Because of slower absorption, death may be more delayed after the ingestion of cyanide salts, but the critical events sdl occur within the first hour. Two other sources of cyanide have been responsible for human poisoning: the naturally occurring amygdalin and the drug nitroprusside. Amygdalin is a cyanogenic glycoside found in apricot, peach, and similar fruit pits and in sweet almonds (Sayre and Kaymakcalan,Возможный контакт
Sodium and potassium cyanides are used primarily in the extraction of ores, electroplating, metal treatment, and various manufacturing processes. Iodine cyanide: Used generally for destroying all lower forms of life; in taxidermy to preserve insects, etc.Канцерогенность
There is no evidence that exposure to cyanide causes cancer. USEPA lists cyanide as not classifiable with respect to its potential to cause cancer in humans. However, certain compounds included in the cyanide group may be human carcinogens. For example, acrylonitrile has been judged to be a probable human carcinogen. Details of these effects are provided in the appropriate sections where individual compounds are discussed.Перевозки
UN1588 Cyanides, inorganic, solid, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials. UN1935Несовместимости
Soluble in water; slow decomposition releases highly toxic and flammable hydrogen cyanide gas. The aqueous solution of potassium and sodium cyanide are highly corrosive, and strong bases. KCN and NaCN react violently with acids, releasing highly flammable hydrogen cyanide. Potassium and sodium cyanide are incompatible with strong oxidizers (such as acids, acid salts; chlorates, nitrates), organic anhydrides; isocyanates, alkylene oxides; epichlorohydrin, aldehydes, alcohols, glycols, phenols, cre- sols, caprolactum. Attacks aluminum, copper, zinc in the presence of moisture. KCN and NaCN absorb moisture from the air forming a corrosive syrup.Утилизация отходов
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform to EPA regulations governing storage, transportation, treatment, and waste disposal. In accordance with 40CFR165, follow recommendations for the disposal of pesticides and pesticide containers. Must be disposed properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office. Add strong alkaline hypochlorite and react for 24 hours. Then flush to sewer with large volumes of water .Метил-4- (цианометил) бензоат запасные части и сырье
сырьё
запасной предмет
Метил-4- (цианометил) бензоат поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86-86-13583358881 +8618560316533 |
China | 3094 | 58 | |
| +86-85511178; +86-85511178; |
China | 35425 | 58 | |
| +86-89586680 +86-13289823923 |
China | 8670 | 58 | |
| China | 12984 | 57 |