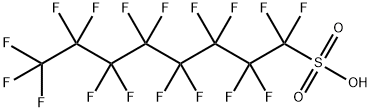
Perfluorooctanesulfonic acid
- русский язык имя
- английское имяPerfluorooctanesulfonic acid
- CAS №1763-23-1
- CBNumberCB0745383
- ФормулаC8HF17O3S
- мольный вес500.13
- EINECS217-179-8
- номер MDLMFCD00042454
- файл Mol1763-23-1.mol
| Температура плавления | 90 °C |
| Температура кипения | 260 °C |
| плотность | 1.25 |
| Fp | 11 °C |
| температура хранения | 2-8°C |
| растворимость | Ethanol: 10 mg/ml |
| пка | -3.27±0.50(Predicted) |
| БРН | 1813859 |
| Справочник по базе данных CAS | 1763-23-1(CAS DataBase Reference) |
| FDA UNII | 9H2MAI21CL |
| Предложение 65 Список | Perfluorooctane sulfonate (PFOS) |
| Система регистрации веществ EPA | Perfluorooctane sulfonic acid (1763-23-1) |
| UNSPSC Code | 85151701 |
| NACRES | NA.24 |
| Коды опасности | C,N,T,F | |||||||||
| Заявления о рисках | 34-57-51/53-22-39/23/24/25-23/24/25-11-64-48/25-40-20/22-61 | |||||||||
| Заявления о безопасности | 26-36/37/39-45-61-36/37-16-7-53 | |||||||||
| РИДАДР | UN 3265 8/PG 2 | |||||||||
| WGK Германия | - | |||||||||
| RTECS | RG9701600 | |||||||||
| Примечание об опасности | Corrosive | |||||||||
| кода HS | 29049090 | |||||||||
| Банк данных об опасных веществах | 1763-23-1(Hazardous Substances Data) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)



-
сигнальный язык
опасность
-
вредная бумага
H302:Вредно при проглатывании.
H372:Поражает органы в результате многократного или продолжительного воздействия.
H351:Предполагается, что данное вещество вызывает раковые заболевания.
H360D:Может отрицательно повлиять на неродившегося ребенка.
H362:Может причинить вред детям, находящимся на грудном вскармливании.
H411:Токсично для водных организмов с долгосрочными последствиями.
-
оператор предупредительных мер
P201:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P202:Перед использованием ознакомиться с инструкциями по технике безопасности.
P263:Избегать контакта в период беременности и грудного вскармливания.
P273:Избегать попадания в окружающую среду.
P301+P312:ПРИ ПРОГЛАТЫВАНИИ: Обратиться за медицинской помощью при плохом самочувствии.
P308+P313:ПРИ подозрении на возможность воздействия обратиться за медицинской помощью.
Perfluorooctanesulfonic acid химические свойства, назначение, производство
Описание
Perfluorooctanesulfonic acid or perfluorooctane sulfonate (PFOS), is a man-made fluorosurfactant and global pollutant. It is a very useful catalyst in chemical industry. It can catalyze the Intramolecular Hydroamination of Olefinic Sulfonamides in Fluorous Biphase System (FBS)1. It can also catalyze ring-opening reactions of methylenecyclopropanes with aromatic amines, sulfonamides and alcohols in supercritical carbon dioxide2. It is useful in the nitroaldol reaction in fluorous media, which is an important improvement of the chemo-selective addition of aldehyde3.Химические свойства
Perfluorooctane sulfonate can exist as a liquid or a powder. It is soluble in water (NTP, 2013). Perfluorooctane sulfonate has the ability to react with strong oxidizing agents. Upon decomposition, PFOS can form carbon oxides, sulfur oxides, and hydrogen fluoride. Additional information related to the physical and chemical properties of PFOS are not currently available.Использование
Perfluorooctane sulfonate (PFOS, 1-octanesulfonic acid, heptadecafluorooctanesulfonic acid, perfluorooctanesulfonic acid, 1-perfluorooctanesulfonic acid) is an eight-carbon compound in the perfluoroalkyl family of chemicals. Perfluorooctane sulfonate is used in a variety of applications, including textiles, leather products, cleaning products, and pesticides. Its main use was as a stain repellent on carpet, furniture, and other consumer products. 3M was the primary manufacturer of the chemical until it voluntarily phased it out in 2002 (USEPA, 2012). Perfluorooctane sulfonate is not manufactured any longer in the United States, but it can be imported for use in specific applications.прикладной
Heptadecafluorooctanesulfonic acid may be used as an analytical reference standard for the determination of the analyte in water samples using solid-phase microextraction combined with high-performance liquid chromatography with electrospray-ion trap mass spectrometry.Определение
ChEBI: Perfluorooctane-1-sulfonic acid is a perfluoroalkanesulfonic acid that is octane-1-sulfonic acid in which all seventeen of the hydrogens that are attached to carbons hvae been replaced by fluorines. It has a role as an antilipemic drug and a persistent organic pollutant. It is functionally related to an octane-1-sulfonic acid.Общее описание
Heptadecafluorooctanesulfonic acid belongs to the class of perfluorinated alkyl acids and is emerging as persistent organic contaminants in the environment.Влияние на здоровье
Exposure to unsafe levels of PFOA/PFOS concentrations through drinking water may result in health effects including developmental effects to fetuses during pregnancy, cancer, liver effects, immune effects and thyroid effects.Экологические соображения
Perfluorooctane sulfonic acid (PFOS) is one of a group of related chemicals known as perfluorinated alkylated substances (PFAS). These are also called perfluorochemicals (PFCs). This group of chemicals is commonly used in a wide range of industrial processes and is found in many consumer products. PFOS can enter groundwater from multiple sources, including sewage treatment plants, industrial sites, landfills, and places where it is used in firefighting foam such as airports, firefighter training sites, and military installations. Fish and shellfish can take up PFOS from water contaminated with the chemical.использованная литература
[1] YAN YIN Gang Z. Heptadecafluorooctanesulfonic acid (C8F17SO3H) catalyzed intramolecular hydroamination of olefinic sulfonamides in fluorous biphase system (FBS)[J]. Journal of Fluorine Chemistry, 2007, 128 1: Pages 40-45. DOI:10.1016/j.jfluchem.2006.09.012.[1] SHI M, CHEN Y, XU B, et al. Heptadecafluorooctanesulfonic acid catalyzed ring opening reactions of methylenecyclopropanes with aromatic amines, sulfonamides and alcohols in supercritical carbon dioxide[J]. Green Chemistry, 2003, 1: 85-88. DOI:10.1039/B210988C.
https://www.sciencedirect.com/science/article/pii/S1566736707001240
https://www.p65warnings.ca.gov/fact-sheets/pfos-perfluorooctane-sulfonate-or-perfluorooctane-sulfonic-acid
https://19january2021snapshot.epa.gov/sites/static/files/2017-12/documents/ffrrofactsheet_contaminants_pfos_pfoa_11-20-17_508_0.pdf
https://www.health.state.mn.us/communities/environment/risk/docs/guidance/gw/pfosinfo.pdf
[1] PING QI. Aquatic predicted no-effect-concentration derivation for perfluorooctane sulfonic acid[J]. Environmental Toxicology and Chemistry, 2011, 30 4: 836-842. DOI:10.1002/etc.460.
Perfluorooctanesulfonic acid поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| +86-0571-85586753; +8613336034509 |
China | 1376 | 60 | |
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 | |
| 86-13657291602 | CHINA | 22963 | 58 | |
| +86-023-6139-8061 +86-86-13650506873 |
China | 39894 | 58 | |
| 18503026267 | CHINA | 9636 | 58 | |
| +86-021-61551413 +8618813727289 |
China | 5738 | 58 | |
| +86-0551-65418671 +8618949823763 |
China | 34563 | 58 | |
| +8615255079626 | China | 23541 | 58 | |
| +8615986615575 | China | 20342 | 58 |